Introduction
Spider nevus is a common sign of liver cirrhosis [1–3]. Spider nevus is so named because of its spider-like appearance in which there is a central red arteriole and radiating thin-walled vessels resembling the body and legs of a spider, respectively. In the general population, it has been reported that the presence of spider nevus is associated with thyrotoxicosis and excessive estrogen, such as pregnancy and oral contraceptives. In cirrhotic patients, the underlying mechanisms of spider nevus include the disturbance of sex hormones (i.e., higher ratio of estradiol to testosterone, increased level of luteinizing hormone, and decreased level of testosterone in male patients) [4–6], angiogenesis (i.e., elevated expression of vascular endothelial growth factor and fibroblast growth factor) [7], vasodilation (increased level of substance P) [8], alcohol abuse [4], hyperdynamic circulation state [9], and liver dysfunction [4, 5]. Evidence suggests that spider nevus can predict the grade of liver fibrosis in patients with chronic liver diseases [10–12], and is closely associated with the likelihood of hepatopulmonary syndrome in patients with portal hypertension [13, 14].
Subcutaneous collateral vessels of the chest/abdominal wall are also frequently observed in cirrhotic patients [15]. In the setting of portal hypertension, paraumbilical vein dilation further leads to the occurrence of subcutaneous collateral vessels of the chest/abdominal wall and even caput medusae [16, 17]. Sometimes, the subcutaneous collateral vessel of the abdominal wall acts as the drainage vessel of intra-abdominal varices, such as stomal and jejunal varices [18, 19]. However, the clinical significance of subcutaneous collateral vessels of the chest/abdominal wall in liver cirrhosis remains unclear.
We conducted a prospective observational study to analyze the impact of spider nevus and subcutaneous collateral vessel of the chest/abdominal wall on the outcomes of patients with liver cirrhosis.
Material and methods
This was a prospective observational study. The study protocol was approved by the Medical Ethical Committee of our hospital. The human study complied with the Declaration of Helsinki. The approval number was No. k(2015)06. The study was also registered in the website clinicaltrial.gov. The register number was NCT02468479. The inclusion criteria were as follows: 1) patients with a diagnosis of liver cirrhosis; and 2) agreement to perform the physical examinations. The exclusion criteria were as follows: 1) a repeated admission; and 2) a confirmed diagnosis of malignancy. The primary endpoint was survival.
Liver cirrhosis was diagnosed based on the clinical symptoms related to liver diseases and portal hypertension, biochemical laboratory tests, liver stiffness measurement, and hepatic ultrasound, computed tomography, and/or magnetic resonance images [20]. If necessary, a liver biopsy was performed. Grades of ascites and hepatic encephalopathy were based on the practice guidelines [21, 22]. Management of liver cirrhosis and portal hypertension related complications was based on the current practice guidelines [21–24].
At admission, three investigators (RW, YP, and XQ) performed the physical examinations for all eligible patients. In the case of uncertainty, they would discuss it with two primary investigators (HL and XG). According to the number of spider nevi of the chest/abdominal wall, they were divided into 0, 1–2, 3–4, and ≥ 5. According to the location of spider nevi, they were divided into chest wall alone, abdominal wall alone, and both chest and abdominal walls. According to the location of subcutaneous collateral vessels of the chest/abdominal wall, they were divided into chest wall alone, abdominal wall alone, and both chest and abdominal walls. Other baseline data were collected about the demographic profile, major clinical symptoms, etiology of liver cirrhosis, white blood cells, platelet count, hemoglobin, and hepatic, renal, and coagulation function. Child-Pugh and MELD scores were calculated according to the relevant formulae [25, 26].
Patients were followed until lost to follow-up, death, or December 31, 2016. Follow-up data were prospectively collected by two investigators (RW and XQ) from the re-admission records and by telephone contacts.
Statistical analysis
Statistical analyses were performed using SPSS statistics 17.0.0 and MedCalc 11.4.2.0 software. Continuous data were expressed as the mean ± standard deviation and median (range) and compared using the independent t test or non-parametric Mann-Whitney U test. Categorical data were expressed as frequency (percentage) and compared using the Pearson χ2 test or Fisher’s exact test. Cumulative risk was estimated by Kaplan-Meier curve analysis and compared by the log-rank test. A two-sided p-value of < 0.05 was considered statistically significant.
Results
Patients
A total of 198 patients were included in the prospective study between June 2015 and May 2016. Patient characteristics are shown in Table I. The majority of patients were male (64.6%), had a history of alcohol abuse (39.9%) and hepatitis B virus infection (32.3%), and were in Child-Pugh class B (51.3%). The in-hospital mortality was 2.02% (4/198). Two patients underwent liver transplantation during follow-up. The overall mortality was 14.14% (28/198) during a mean follow-up duration of 327.5 ±152.97 days (median: 350 days; range: 6–567).
Table I
Baseline characteristics of 198 patients
| Variables | No. pts. with available data | Mean ± SD or Frequency (percentage) | Median (range) |
|---|
| Sex (male/female), n (%) | 198 | 128 (64.65)/70 (35.35) | |
| Age [years] | 198 | 56.95 ±11.41 | 56.38 (26.76–88.10) |
| Etiology of liver diseases, n (%): | | | |
| Hepatitis B virus infection | 198 | 64 (32.30) | |
| Hepatitis C virus infection | 198 | 18 (9.09) | |
| Alcohol abuse | 198 | 79 (39.90) | |
| Drug-induced liver injury | 198 | 10 (5.05) | |
| Autoimmune liver disease | 198 | 9 (4.55) | |
| Previous gastrointestinal bleeding, n (%) | 198 | 106 (53.54) | |
| Hepatic encephalopathy at admission, n (%) | 198 | 16 (8.08) | |
| Gastrointestinal bleeding at admission, n (%) | 198 | 106 (53.54) | |
| Jaundice at admission, n (%) | 198 | 36 (18.18) | |
| Ascites at admission (no/mild/moderate-severe), n (%) | 198 | 80 (40.40)/39 (19.70)/79 (39.90) | |
| Spider nevus of chest/abdominal wall, n (%): | 198 | 93 (46.97) | |
| Number (1–2/3–4/≥ 5) | 93 | 46 (49.46)/20 (21.51)/27 (29.03) | |
| Location (chest alone/abdomen alone/both chest and abdomen) | 93 | 91 (97.85)/0 (0.00)/2 (2.15) | |
| Subcutaneous collateral vessel of chest/abdominal wall, n (%): | 198 | 59 (29.80) | |
| Location (chest alone/abdomen alone/both chest and abdomen) | 59 | 10 (16.95)/33 (55.93)/16 (27.12) | |
| Laboratory tests: | | | |
| Red blood cells [× 1012/l] | 197 | 3.14 ±0.83 | 3.04 (1.42–5.64) |
| Hemoglobin [g/l] | 197 | 92.18 ±27.71 | 91.00 (37.00–170.00) |
| White blood cells [× 109/l] | 197 | 5.14 ±3.39 | 4.30 (1.10–23.10) |
| Platelets [× 109/l] | 197 | 91.94 ±59.00 | 75.00 (11.00–346.00) |
| Alanine aminotransferase [U/l] | 195 | 41.62 ±60.47 | 25.52 (4.61–590.00) |
| Aspartate aminotransferase [U/l] | 195 | 62.34 ±80.89 | 37.66 (6.97–719.84) |
| Alkaline phosphatase [U/l] | 195 | 126.33 ±105.26 | 95.00 (33.00–850.45) |
| γ-Glutamyl transpeptidase [U/l] | 195 | 115.38 ±176.31 | 46.16 (9.00–981.00) |
| Total bilirubin [µmol/l] | 195 | 37.06 ±46.01 | 23.40 (6.40–319.90) |
| Prothrombin time [s] | 193 | 16.55 ±3.31 | 15.70 (11.30–31.60) |
| International normalized ratio | 191 | 1.38 ±0.38 | 1.29 (0.83–3.03) |
| Activated partial thromboplastin time [s] | 192 | 40.54 ±5.99 | 39.65 (29.20–57.80) |
| Albumin [g/l] | 194 | 30.35 ±6.84 | 29.70 (16.10–48.10) |
| Serum sodium [mmol/l] | 196 | 138.85 ±3.91 | 138.40 (120.70–151.60) |
| Serum potassium [mmol/l] | 196 | 3.91 ±0.46 | 3.87 (2.48–5.60) |
| Blood urea nitrogen [mmol/l] | 194 | 7.57 ±5.88 | 5.72 (1.71–47.21) |
| Serum creatinine [µmol/l] | 194 | 78.44 ±65.04 | 65.41 (32.14–533.70) |
| Child-Pugh score | 189 | 7.89 ±2.09 | 8.00 (5.00–14.00) |
| Child-Pugh class A/B/C, n (%) | 189 | 55 (29.10)/97 (51.32)/37 (19.58) | |
| MELD score | 187 | 8.64 ±6.02 | 7.70 (–2.38–28.02) |
| In-hospital death, n (%) | 198 | 4 (2.02) | |
| Overall death, n (%) | 198 | 28 (14.14) | |
Spider nevus of chest/abdominal wall
The prevalence of spider nevus of the chest/abdominal wall was 47% (93/198). Of the 93 patients with spider nevi, 49.46% (46/93), 21.51% (20/93), and 29.03% (27/93) had 1–2, 3–4, and ≥ 5 spider nevi, respectively. The location of the spider nevus was the chest wall alone in nearly all patients (97.85%, 91/93).
Patients with spider nevi of the chest/abdominal wall had significantly higher proportions of male sex, alcohol abuse, subcutaneous collateral vessel of the chest/abdominal wall, and Child-Pugh classes B and C, significantly higher hemoglobin, white blood cells, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total bilirubin, activated partial thromboplastin time, Child-Pugh score, and MELD score, and significantly lower sodium, and were significantly younger than those without spider nevi of the chest/abdominal wall (Table II). The cumulative survival was not significantly different between patients with and without spider nevi of the chest/abdominal wall (p = 0.951) (Figure 1 A).
Table II
Comparison between patients with and without spider nevus of chest/abdominal wall
| Variables | With spider nevus of chest/abdominal wall | Without spider nevus of chest/abdominal wall | P-value |
|---|
| N | Mean ± SD or Frequency (percentage) | Median (range) | N | Mean ± SD or Frequency (percentage) | Median (range) |
|---|
| Sex (male/female), n (%) | 93 | 68 (73.12)/25 (26.88) | | 105 | 60 (57.14)/45 (42.86) | | 0.028 |
| Age (years] | 93 | 54.19 ±10.03 | 53.83 (27.08–82.34) | 105 | 59.39 ±12.03 | 59.89 (26.76–88.10) | 0.001 |
| Etiology of liver diseases, n (%): | | | | | | | |
| Hepatitis B virus infection | 93 | 25 (26.88) | | 105 | 39 (37.14) | | 0.104 |
| Hepatitis C virus infection | 93 | 5 (5.38) | | 105 | 13 (12.38) | | 0.099 |
| Alcohol abuse | 93 | 51 (54.84) | | 105 | 28 (26.67) | | < 0.001 |
| Drug-induced liver injury | 93 | 3 (3.23) | | 105 | 7 (6.67) | | 0.436 |
| Autoimmune liver disease | 93 | 4 (4.30) | | 105 | 5 (4.76) | | 0.852 |
| Previous gastrointestinal bleeding, n (%) | 93 | 42 (45.16) | | 105 | 64 (60.95) | | 0.038 |
| Hepatic encephalopathy at admission, n (%) | 93 | 43 (46.24) | | 105 | 63 (60.00) | | 0.073 |
| Gastrointestinal bleeding at admission, n (%) | 93 | 20 (21.51) | | 105 | 16 (15.24) | | 0.339 |
| Jaundice at admission, n (%) | 93 | 2 (2.15) | | 105 | 2 (1.90) | | 0.701 |
| Ascites at admission (no/mild/moderate-severe), n (%) | 93 | 31 (33.33)/17 (18.28)/45 (48.39) | | 105 | 49 (46.67)/22 (20.95)/34 (32.38) | | 0.063 |
| Subcutaneous collateral vessel of chest/abdominal wall, n (%): | 93 | 35 (37.63)/58 (62.37) | | 105 | 24 (22.86)/81 (77.14) | | 0.035 |
| Location (chest alone/abdomen alone/both chest and abdomen) | 35 | 5 (14.29)/17 (48.57)/13 (37.14) | | 24 | 5 (20.83)/16 (66.67)/3 (12.50) | | 0.112 |
| Laboratory tests: | | | | | | | |
| Red blood cells [× 1012/l] | 93 | 3.20 ±0.83 | 3.11 (1.69–5.06) | 104 | 3.09 ±0.83 | 2.97 (1.42–5.64) | 0.356 |
| Hemoglobin [g/l] | 93 | 97.60 ±27.95 | 96.00 (47.00–170.00) | 104 | 87.33 ±26.71 | 83.00 (37.00–164.00) | 0.012 |
| White blood cells [× 109/l] | 93 | 5.27 ±2.82 | 4.50 (1.70–16.00) | 103 | 5.04 ±3.86 | 4.00 (1.10–23.10) | 0.047 |
| Platelets [× 109/l] | 93 | 95.55 ±57.36 | 81.00 (19.00–282.00) | 104 | 88.71 ±60.53 | 73.50 (11.00–346.00) | 0.316 |
| Alanine aminotransferase [U/l] | 92 | 48.64 ±77.82 | 28.50 (6.24–590.00) | 103 | 35.36 ±38.32 | 22.07 (4.61–192.51) | 0.005 |
| Aspartate aminotransferase [U/l] | 92 | 77.72 ±101.85 | 47.07 (13.54–719.84) | 103 | 48.60 ±52.79 | 31.20 (6.97–300.70) | < 0.001 |
| Alkaline phosphatase [U/l] | 92 | 131.27 ±95.28 | 101.74 (33.00–649.21) | 103 | 121.91 ±113.72 | 85.59 (35.36–850.45) | 0.049 |
| γ-Glutamyl transpeptidase [U/l] | 92 | 153.41 ±204.87 | 73.60 (9.00–981.00) | 103 | 81.41 ±138.59 | 34.65 (9.67–797.00) | < 0.001 |
| Total bilirubin [µmol/l] | 92 | 48.13 ±59.90 | 28.95 (6.40–319.90) | 103 | 27.18 ±24.81 | 20.10 (7.10–179.20) | 0.004 |
| Prothrombin time [s] | 93 | 16.97 ±3.54 | 15.90 (11.70–31.60) | 100 | 16.15 ±3.06 | 15.55 (11.30–29.70) | 0.080 |
| International normalized ratio | 92 | 1.43 ±0.40 | 1.31 (0.89–3.03) | 99 | 1.34 ±0.35 | 1.25 (0.83–3.00) | 0.065 |
| Activated partial thromboplastin time [s] | 93 | 41.43 ±6.08 | 40.30 (29.40–54.50) | 99 | 39.71 ±5.81 | 38.60 (29.20–57.80) | 0.040 |
| Albumin [g/l] | 92 | 29.84 ±6.91 | 29.40 (16.10–47.20) | 102 | 30.80 ±6.78 | 30.20 (17.20–48.10) | 0.404 |
| Serum sodium [mmol/l] | 92 | 136.67 ±4.18 | 137.50 (120.70–144.60) | 104 | 138.89 ±3.34 | 139.40 (130.40–151.60) | < 0.001 |
| Serum potassium [mmol/l] | 92 | 3.90 ±0.45 | 3.85 (2.73–4.88) | 104 | 3.92 ±0.47 | 3.87 (2.48–5.60) | 0.987 |
| Blood urea nitrogen [mmol/l] | 93 | 7.43 ±6.59 | 5.50 (1.90–47.21) | 101 | 7.69 ±5.16 | 6.03 (1.71–30.40) | 0.134 |
| Serum creatinine [µmol/l] | 93 | 81.90 ±72.93 | 65.69 (32.14–533.70) | 101 | 75.25 ±57.00 | 65.30 (34.90–440.06) | 0.651 |
| Child-Pugh score | 91 | 8.35 ±2.19 | 8.00 (5.00–14.00) | 98 | 7.47 ±1.91 | 7.00 (5.00–13.00) | 0.005 |
| Child-Pugh class A/B/C, n (%) | 91 | 17 (18.68)/50 (54.95)/24 (26.37) | | 98 | 38 (38.78)/47 (47.96)/13 (13.26) | | 0.004 |
| MELD score | 91 | 9.91 ±6.41 | 8.86 (–1.42 – 28.02) | 96 | 7.43 ±5.40 | 5.66 (–2.38 – 22.05) | 0.008 |
| In-hospital death, n (%) | 93 | 2 (2.15) | | 105 | 2 (1.90) | | 0.701 |
| Overall death, n (%) | 93 | 13 (13.98) | | 105 | 15 (14.29) | | 0.887 |
Figure 1
Kaplan-Meir curve regarding the impact of spider nevus on cumulative survival of cirrhotic patients. A – Patients with vs. without spider nevus. B – Patients with 1–2 vs. 3–4 vs. ≥ 5 spider nevi of chest/abdominal wall
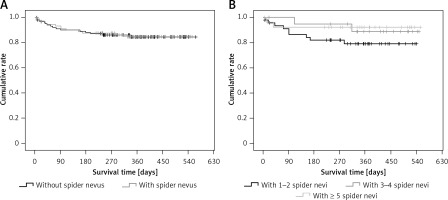
Proportions of male sex and alcohol abuse were significantly different among patients with different numbers of spider nevi of the chest/abdominal wall (Table III). The cumulative survival was not significantly different among them (p = 0.151) (Figure 1 B).
Table III
Comparison among patients with different number of spider nevi
| Variables | With 1–2 spider nevi of chest/abdominal wall | With 3–4 spider nevi of chest/abdominal wall | With ≥ 5 spider nevi of chest/abdominal wall | P-value |
|---|
| N | Mean ± SD or Frequency (percentage) | Median (range) | N | Mean ± SD or Frequency (percentage) | Median (range) | N | Mean ± SD orFrequency (percentage) | Median (range) | |
|---|
| Sex (male/female), n (%) | 46 | 28 (60.87)/18 (39.13) | | 20 | 15 (75.00)/5 (25.00) | | 27 | 25 (92.59)/2 (7.41) | | 0.013 |
| Age [years] | 46 | 56.43 ±11.52 | 56.62 (27.08–82.34) | 20 | 52.48 ±7.91 | 53.28 (32.45–66.89) | 27 | 51.63 ±7.93 | 35.71 (35.74–69.03) | 0.098 |
| Etiology of liver diseases, n (%): | | | | | | | 27 | | | |
| Hepatitis B virus infection | 46 | 13 (28.26) | | 20 | 7 (35.00) | | 27 | 5 (18.52) | | 0.125 |
| Hepatitis C virus infection | 46 | 4 (8.70) | | 20 | 1 (5.00) | | 27 | 0 (0.00) | | 0.371 |
| Alcohol abuse | 46 | 19 (41.30) | | 20 | 10 (50.00) | | 27 | 22 (81.48) | | 0.004 |
| Drug-induced liver injury | 46 | 2 (4.35) | | 20 | 0 (0.00) | | 27 | 1 (3.70) | | 0.647 |
| Autoimmune liver disease | 46 | 2 (4.35) | | 20 | 2 (10.00) | | 27 | 0 (0.00) | | 0.248 |
| Previous gastrointestinal bleeding, n (%) | 46 | 22 (47.83) | | 20 | 8 (40.00) | | 27 | 12 (44.44) | | 0.838 |
| Hepatic encephalopathy at admission, n (%) | 46 | 5 (10.87) | | 20 | 2 (10.00) | | 27 | 4 (14.81) | | 0.846 |
| Gastrointestinal bleeding at admission, n (%) | 46 | 24 (52.17) | | 20 | 9 (45.00) | | 27 | 10 (37.04) | | 0.453 |
| Jaundice at admission, n (%) | 46 | 10 (21.74) | | 20 | 3 (15.00) | | 27 | 7 (25.93) | | 0.665 |
| Ascites at admission (no/mild/moderate-severe), n (%) | 46 | 15 (32.61)/7 (15.22)/24 (52.17) | | 20 | 6 (30.00)/4 (20.00)/10 (50.00) | | 27 | 10 (37.03)/6 (22.22)/11 (40.74) | | 0.885 |
| Subcutaneous collateral vessel of chest/abdominal wall, n (%): | 46 | 13 (28.26)/33 (71.74) | | 20 | 10 (50.00)/10 (50.00) | | 27 | 12 (44.44)/15 (55.56) | | 0.169 |
| Location (chest alone/abdomen alone/both chest and abdomen) | 13 | 0 (0.00)/7 (53.85)/6 (46.15) | | 10 | 2 (20.00)/4 (40.00)/4 (40.00) | | 12 | 3 (25.00)/6 (50.00)/3 (25.00) | | 0.398 |
| Laboratory tests: | | | | | | | | | | |
| Red blood cells [× 1012/l] | 46 | 3.18 ±0.86 | 3.05 (1.69–5.06) | 20 | 3.32 ±0.81 | 3.18 (2.09–4.81) | 27 | 3.15 ±0.80 | 3.13 (1.74–5.02) | 0.755 |
| Hemoglobin [g/l] | 46 | 91.91 ±26.75 | 83.50 (54.00–149.00) | 20 | 102.80 ±32.74 | 100.00 (47.00–151.00) | 27 | 103.44 ±25.08 | 101.00 (52.00–170.00) | 0.152 |
| White blood cells [× 109/l] | 46 | 5.54 ±3.15 | 4.45 (1.70–14.50) | 20 | 4.78 ±1.28 | 4.70 (2.30–6.70) | 27 | 5.18 ±3.06 | 4.30 (1.80–16.00) | 0.588 |
| Platelets [× 109/l] | 46 | 93.67 ±56.56 | 74.00 (29.00–282.00) | 20 | 112.40 ±67.45 | 95.50 (36.00–282.00) | 27 | 86.26 ±49.67 | 84.00 (19.00–192.00) | 0.290 |
| Alanine aminotransferase [U/l] | 45 | 46.16 ±70.77 | 25.85 (13.42–466.75) | 20 | 61.61 ±125.73 | 29.87 (6.24–590.00) | 27 | 43.15 ±31.60 | 41.89 (12.54–170.79) | 0.697 |
| Aspartate aminotransferase [U/l] | 45 | 72.22 ±108.88 | 39.85 (18.16–719.84) | 20 | 82.24 ±136.02 | 47.47 (13.54–645.00) | 27 | 83.53 ±51.22 | 72.00 (15.35–166.49) | 0.881 |
| Alkaline phosphatase [U/l] | 45 | 121.17 ±107.03 | 94.64 (39.17–649.21) | 20 | 145.25 ±98.69 | 116.00 (47.00–444.88) | 27 | 137.75 ±69.28 | 118.46 (33.00–386.00) | 0.593 |
| Gamma-glutamyl transpeptidase [U/l] | 45 | 117.82 ±191.67 | 60.66 (9.00–929.28) | 20 | 191.77 ±266.82 | 67.74 (10.00–981.00) | 27 | 184.33 ±168.48 | 94.00 (12.18–552.26) | 0.265 |
| Total bilirubin [µmol/l] | 45 | 47.24 ±59.91 | 26.00 (7.70–316.90) | 20 | 35.63 ±30.14 | 27.55 (8.80–138.00) | 27 | 58.86 ±74.70 | 37.50 (6.40–319.90) | 0.422 |
| Prothrombin time [s] | 46 | 17.07 ±3.82 | 15.85 (11.70–31.60) | 20 | 16.40 ±3.52 | 15.80 (12.30–28.90) | 27 | 17.23 ±3.10 | 15.90 (13.00–25.20) | 0.703 |
| International normalized ratio | 45 | 1.44 ±0.43 | 1.30 (0.89–3.03) | 20 | 1.37 ±0.41 | 1.28 (0.93–2.89) | 27 | 1.45 ±0.35 | 1.31 (0.97–2.36) | 0.720 |
| Activated partial thromboplastin time [s] | 46 | 40.90 ±6.33 | 40.30 (29.40–54.50) | 20 | 40.16 ±5.15 | 38.50 (32.70–52.80) | 27 | 43.29 ±6.07 | 43.10 (34.30–54.50) | 0.154 |
| Albumin [g/l] | 46 | 29.72 ±7.03 | 29.15 (17.80–47.20) | 19 | 31.22 ±5.86 | 30.10 (19.80–42.40) | 27 | 29.10 ±7.48 | 29.30 (16.10–43.40) | 0.587 |
| Serum sodium [mmol/l] | 46 | 137.05 ±3.57 | 137.20 (129.80–144.60) | 19 | 137.54 ±3.86 | 138.40 (128.00–143.40) | 27 | 135.43 ±5.13 | 137.50 (120.70–141.20) | 0.168 |
| Serum potassium [mmol/l] | 46 | 3.90 ±0.41 | 3.88 (3.01–4.85) | 19 | 3.89 ±0.48 | 3.81 (2.73–4.66) | 27 | 3.91 ±0.50 | 3.94 (2.96–4.88) | 0.990 |
| Blood urea nitrogen [mmol/l] | 46 | 7.85 ±5.67 | 6.13 (2.45–32.70) | 20 | 6.37 ±4.75 | 5.24 (2.24–25.59) | 27 | 7.50 ±8.96 | 4.86 (1.90–47.21) | 0.710 |
| Serum creatinine [µmol/l] | 45 | 75.74 ±48.06 | 64.55 (32.14–317.30) | 20 | 89.17 ±89.74 | 70.19 (44.00–463.47) | 27 | 87.01 ±93.83 | 64.20 (41.76–533.70) | 0.723 |
| Child-Pugh score | 45 | 8.40 ±2.26 | 8.00 (5.00–14.00) | 19 | 8.05 ±1.47 | 8.00 (5.00–10.00) | 27 | 8.48 ±2.53 | 8.00 (5.00–13.00) | 0.794 |
| Child-Pugh class A/B/C, n (%) | 45 | 8 (17.78)/26 (57.78)/11 (24.44) | | 19 | 2 (10.53)/14 (73.68)/3 (15.79) | | 27 | 7 (25.92)/10 (37.04)/10 (37.04) | | 0.175 |
| MELD score | 44 | 9.52 ±6.44 | 8.89 (–1.42–22.78) | 20 | 9.41 ±6.41 | 8.15 (0.41–28.02) | 27 | 10.92 ±6.47 | 9.16 (–1.15 – 23.47) | 0.624 |
| In-hospital death, n (%) | 46 | 1 (2.17) | | 20 | 0 (0.00) | | 27 | 1 (3.70) | | 0.688 |
| Overall death, n (%) | 46 | 9 (19.57) | | 20 | 2 (10.00) | | 27 | 2 (7.41) | | 0.297 |
Subcutaneous collateral vessel of chest/abdominal wall
The prevalence of subcutaneous collateral vessel of the chest/abdominal wall was 29.8% (59/198). The location of the subcutaneous collateral vessel was the chest wall alone, the abdominal wall alone, and both chest and abdominal walls in 16.9% (10/59), 55.9% (33/59), and 27.1% (16/59), respectively.
Patients with subcutaneous collateral vessels of the chest/abdominal wall had significantly higher proportions of moderate to severe ascites at admission, spider nevus, and Child-Pugh class C, significantly higher alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyl transpeptidase, total bilirubin, Child-Pugh score, and MELD score, significantly lower proportions of previous history of gastrointestinal bleeding and gastrointestinal bleeding at admission, and significantly lower sodium than those without subcutaneous collateral vessel of chest/abdominal wall (Table IV). The cumulative survival was significantly worse in patients with a subcutaneous collateral vessel of the chest/abdominal wall than in those without a subcutaneous collateral vessel of the chest/abdominal wall (p = 0.018) (Figure 2 A).
Table IV
Comparison between patients with and without subcutaneous collateral vessel of chest/abdominal wall
| Variables | With subcutaneous collateral vessel of chest/abdominal wall | Without subcutaneous collateral vessel of chest/abdominal wall | P-value |
|---|
| N | Mean ± SD or Frequency (percentage) | Median (range) | N | Mean ± SD or Frequency (percentage) | Median (range) |
|---|
| Sex (male/female), n (%) | 59 | 37 (62.71)/22 (37.29) | | 139 | 91 (65.47)/48 (34.53) | | 0.835 |
| Age [years] | 59 | 57.19 ±11.75 | 57.01 (31.96–88.10) | 139 | 56.84 ±11.30 | 56.31 (26.76–82.34) | 0.713 |
| Etiology of liver diseases, n (%): | | | | | | | |
| Hepatitis B virus infection | 59 | 18 (30.51) | | 139 | 46 (33.09) | | 0.850 |
| Hepatitis C virus infection | 59 | 3 (5.08) | | 139 | 15 (10.79) | | 0.314 |
| Alcohol abuse | 59 | 28 (47.46) | | 139 | 51 (36.69) | | 0.209 |
| Drug-induced liver injury | 59 | 1 (1.69) | | 139 | 9 (6.47) | | 0.294 |
| Autoimmune liver disease | 59 | 2 (3.39) | | 139 | 7 (5.04) | | 0.892 |
| Previous gastrointestinal bleeding, n (%) | 59 | 24 (40.68) | | 139 | 82 (58.99) | | 0.027 |
| Hepatic encephalopathy at admission, n (%) | 59 | 4 (6.78) | | 139 | 12 (8.63) | | 0.879 |
| Gastrointestinal bleeding at admission, n (%) | 59 | 19 (32.20) | | 139 | 87 (62.59) | | < 0.001 |
| Jaundice at admission, n (%) | 59 | 16 (27.12) | | 139 | 20 (14.39) | | 0.055 |
| Ascites at admission (no/mild/moderate-severe), n (%) | 59 | 15 (25.42)/9 (15.25)/35 (59.32) | | 139 | 65 (46.76)/30 (21.58)/44 (31.65) | | 0.001 |
| Spider nevus of chest/abdominal wall, n (%): | 59 | 35 (59.32) | | 139 | 58 (41.73) | | 0.035 |
| Number (1–2/3–4/≥ 5) | 35 | 13 (37.14)/10 (28.57)/12 (34.29) | | 58 | 33 (56.90)/10 (17.24)/15 (25.86) | | 0.169 |
| Location (chest alone/abdomen alone/both chest and abdomen) | 35 | 34 (97.14)/0 (0)/1 (2.86) | | 58 | 57 (98.28)/0 (0.00)/1 (1.02) | | 0.071 |
| Laboratory tests: | | | | | | | |
| Red blood cells [× 1012/l] | 59 | 3.14 ±0.78 | 3.06 (1.42–5.02) | 138 | 3.15 ±0.85 | 3.04 (1.43–5.64) | 0.839 |
| Hemoglobin [g/l] | 59 | 93.92 ±26.08 | 95.00 (38.00–170.00) | 138 | 91.44 ±28.45 | 84.00 (37.00–164.00) | 0.455 |
| White blood cells [× 109/l] | 59 | 5.16 ±3.26 | 4.40 (1.40–16.00) | 138 | 5.13 ±3.46 | 4.30 (1.10–23.10) | 0.810 |
| Platelets [× 109/l] | 59 | 97.02 ±61.34 | 83.00 (11.00–282.00) | 138 | 89.77 ±58.07 | 74.00 (17.00–346.00) | 0.439 |
| Alanine aminotransferase [U/l] | 59 | 42.98 ±37.79 | 30.22 (6.24–192.51) | 136 | 41.04 ±68.12 | 24.41 (4.61–590.00) | 0.046 |
| Aspartate aminotransferase [U/l] | 59 | 70.23 ±56.98 | 52.42 (13.54–300.70) | 136 | 58.91 ±89.27 | 33.27 (6.97–719.84) | 0.003 |
| Alkaline phosphatase [U/l] | 59 | 161.57 ±131.87 | 124.79 (33.00–850.45) | 136 | 111.04 ±87.59 | 86.82 (35.36–649.21) | 0.003 |
| γ-Glutamyl transpeptidase [U/l] | 59 | 137.47 ±175.01 | 74.58 (10.00–929.28) | 136 | 105.80 ±176.65 | 41.26 (9.00–981.00) | 0.009 |
| Total bilirubin [µmol/l] | 59 | 53.34 ±64.62 | 29.20 (7.90–319.90) | 136 | 30.00 ±32.89 | 22.20 (6.40–316.90) | 0.009 |
| Prothrombin time [s] | 59 | 17.06 ±3.61 | 15.70 (12.50–29.70) | 135 | 16.33 ±3.17 | 15.70 (11.30–31.60) | 0.318 |
| International normalized ratio | 58 | 1.44 ±0.41 | 1.29 (0.95–3.00) | 133 | 1.36 ±0.36 | 1.28 (0.83–3.03) | 0.258 |
| Activated partial thromboplastin time [s] | 58 | 42.47 ±6.12 | 41.15 (29.50–56.10) | 134 | 39.71 ±5.76 | 38.50 (29.20–57.80) | 0.004 |
| Albumin [g/l] | 59 | 29.89 ±6.29 | 29.20 (17.20–47.80) | 135 | 30.55 ±7.08 | 30.10 (16.10–481.00) | 0.557 |
| Serum sodium [mmol/l] | 58 | 136.36 ±4.71 | 137.50 (120.70–143.00) | 138 | 138.48 ±3.35 | 138.50 (128.00–151.60) | 0.019 |
| Serum potassium [mmol/l] | 58 | 3.89 ±0.48 | 3.88 (2.73–4.88) | 138 | 3.92 ±0.45 | 3.87 (2.48–5.60) | 0.808 |
| Blood urea nitrogen [mmol/l] | 59 | 7.90 ±6.61 | 5.62 (2.24–32.70) | 135 | 7.42 ±5.54 | 5.82 (1.71–47.21) | 0.858 |
| Serum creatinine [µmol/l] | 59 | 87.89 ±77.69 | 65.81 (32.14–463.47) | 135 | 74.31 ±58.52 | 64.42 (34.90–533.70) | 0.192 |
| Child-Pugh score | 58 | 8.57 ±2.20 | 8.00 (5.00–14.00) | 131 | 7.60 ±1.98 | 7.00 (5.00–14.00) | 0.002 |
| Child-Pugh class A/B/C, n (%) | 58 | 11 (18.97)/29 (50)/18 (31.03) | | 131 | 44 (33.59)/68 (51.91)/19 (14.50) | | 0.014 |
| MELD score | 58 | 10.77 ±6.76 | 9.16 (–1.42–28.02) | 129 | 7.68 ±5.42 | 6.16 (–2.38 – 23.47) | 0.003 |
| In-hospital death, n (%) | 59 | 1 (1.69) | | 139 | 3 (2.16) | | 0.734 |
| Overall death, n (%) | 59 | 13 (22.03) | | 139 | 13 (9.35) | | 0.022 |
Figure 2
Kaplan-Meir curve regarding the impact of subcutaneous collateral vessel of abdominal/chest wall on cumulative survival of cirrhotic patients. A – Patients with versus without subcutaneous collateral vessel of abdominal/chest wall. B – Patients with subcutaneous collateral vessel of chest wall alone versus abdominal wall alone versus both abdominal and chest walls
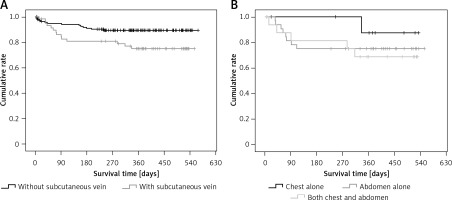
Age, proportion of alcohol abuse, white blood cells, platelets, and gamma-glutamyl transpeptidase were significantly different among patients with different locations of the subcutaneous collateral vessel (Table V). The cumulative survival was not significantly different among them (p = 0.532) (Figure 2 B).
Table V
Comparison among patients with different location of subcutaneous collateral vessel of chest/abdominal wall
| Variables | Chest wall alone | Abdominal wall alone | Both | P-value |
|---|
| N | Mean ± SD or Frequency (percentage) | Median (range) | N | Mean ± SD or Frequency (percentage) | Median (range) | N | Mean ± SD or Frequency (percentage) | Median (range) |
|---|
| Sex (male/female), n (%) | 10 | 5 (50.00)/5 (50.00) | | 33 | 18 (54.55)/15 (45.45) | | 16 | 14 (87.50)/2 (12.50) | | 0.054 |
| Age [years] | 10 | 61.01 ±15.01 | 57.54 (45.17–88.10) | 33 | 59.15 ±11.69 | 62.23 (31.96–78.67) | 16 | 50.77 ±6.70 | 51.31 (39.61–62.71) | 0.034 |
| Etiology of liver diseases, n (%): | | | | | | | | | | |
| Hepatitis B virus infection | 10 | 3 (30.00) | | 33 | 14 (42.42) | | 16 | 1 (6.25) | | 0.099 |
| Hepatitis C virus infection | 10 | 0 (0.00) | | 33 | 1 (3.03) | | 16 | 2 (12.50) | | 0.266 |
| Alcohol abuse | 10 | 3 (30.00) | | 33 | 12 (36.36) | | 16 | 13 (81.25) | | 0.006 |
| Drug-induced liver injury | 10 | 0 (0.00) | | 33 | 1 (3.03) | | 16 | 0 (0.00) | | 0.670 |
| Autoimmune liver disease | 10 | 0 (0.00) | | 33 | 2 (6.06) | | 16 | 0 (0.00) | | 0.442 |
| Previous gastrointestinal bleeding, n (%) | 10 | 6 (60.00) | | 33 | 13 (39.39) | | 16 | 5 (31.25) | | 0.340 |
| Hepatic encephalopathy at admission, n (%) | 10 | 0 (0.00) | | 33 | 2 (6.06) | | 16 | 2 (12.50) | | 0.453 |
| Gastrointestinal bleeding at admission, n (%) | 10 | 4 (40.00) | | 33 | 7 (21.21) | | 16 | 8 (50.00) | | 0.109 |
| Jaundice at admission, n (%) | 10 | 2 (20.00) | | 33 | 8 (24.24) | | 16 | 6 (37.50) | | 0.531 |
| Ascites at admission (no/mild/moderate-severe), n (%) | 10 | 4 (40.00)/3 (30.00)/3 (30.00) | | 33 | 8 (24.24)/5 (15.15)/20 (60.61) | | 16 | 3 (18.75)/1 (6.25)/12 (75.00) | | 0.237 |
| Spider nevus of chest/abdominal wall, n (%): | 10 | 5 (50.00) | | 33 | 17 (51.52) | | 16 | 13 (81.25) | | 0.112 |
| Number (1–2/3–4/≥ 5) | 5 | 0 (0.00)/2 (40.00)/3 (60.00) | | 17 | 7 (41.18)/4 (23.53)/6 (35.29) | | 13 | 6 (46.15)/4 (30.77)/3 (23.08) | | 0.398 |
| Location (chest alone/abdomen alone/both chest and abdomen) | 10 | 5 (50.00)/5 (50.00)/0 (0.00) | | 33 | 16 (48.48)/16 (48.48)/1 (3.03) | | 16 | 3 (18.75)/13 (81.25)/0 (0.00) | | 0.238 |
| Laboratory tests: | | | | | | | | | | |
| Red blood cells [1012/l] | 10 | 3.35 ±0.99 | 3.42 (1.42–4.89) | 33 | 3.27 ±0.76 | 3.11 (2.09–5.02) | 16 | 2.75 ±0.58 | 2.73 (1.69–3.91) | 0.093 |
| Hemoglobin [g/l] | 10 | 97.10 ±29.35 | 100.50 (38.00–131.00) | 33 | 97.91 ±25.75 | 95.00 (55.00–170.00) | 16 | 83.69 ±23.25 | 84.5 (47.00–128.00) | 0.185 |
| White blood cells [× 109/l] | 10 | 3.42 ±1.26 | 3.40 (1.40–5.30) | 33 | 4.63 ±2.57 | 4.20 (1.70–14.00) | 16 | 7.34 ±4.28 | 5.75 (2.90–16.00) | 0.003 |
| Platelets [× 109/l] | 10 | 90.20 ±59.44 | 71.50 (34.00–228.00) | 33 | 82.24 ±42.91 | 73.00 (11.00–171.00) | 16 | 131.75 ±81.88 | 116.5 (22.00–282.00) | 0.025 |
| Alanine aminotransferase [U/l] | 10 | 51.33 ±45.86 | 44.26 (11.77–164.25) | 33 | 45.40 ±42.13 | 30.22 (6.24–192.51) | 16 | 32.78 ±17.41 | 27.57 (13.42–73.00) | 0.416 |
| Aspartate aminotransferase [U/l] | 10 | 76.34 ±59.34 | 64.72 (15.85–211.18) | 33 | 66.99 ±61.31 | 48.46 (13.54–300.70) | 16 | 73.11 ±48.67 | 64.78 (19.64–161.31) | 0.846 |
| Alkaline phosphatase [U/l] | 10 | 210.63 ±164.21 | 142.37 (49.82–542.00) | 33 | 156.70 ±145.44 | 114.00 (33.00–850.45) | 16 | 140.94 ±61.83 | 136.5 (45.00–282.00) | 0.410 |
| γ-Glutamyl transpeptidase [U/l] | 10 | 120.78 ±115.29 | 89.85 (19.22–399.94) | 33 | 92.31 ±92.99 | 47.00 (10.00–349.83) | 16 | 241.04 ±275.49 | 122 (11.00–929.28) | 0.017 |
| Total bilirubin [µmol/l] | 10 | 34.91 ±27.13 | 33.85 (7.90–97.30) | 33 | 52.94 ±64.98 | 24.40 (8.00–281.70) | 16 | 65.68 ±79.42 | 41.6 (10.50–319.90) | 0.432 |
| Prothrombin time [s] | 10 | 16.91 ±3.40 | 15.60 (13.40–25.20) | 33 | 17.01 ±3.90 | 15.70 (12.50–29.70) | 15 | 17.26 ±3.27 | 15.5 (12.80–22.50) | 0.967 |
| International normalized ratio | 10 | 1.42 ±0.38 | 1.28 (1.04–2.36) | 33 | 1.44 ±0.45 | 1.29 (0.95–3.00) | 15 | 1.47 ±0.37 | 1.26 (0.98–2.07) | 0.965 |
| Activated partial thromboplastin time [s] | 10 | 43.67 ±7.35 | 42.60 (34.80–54.50) | 33 | 42.07 ±6.41 | 40.30 (29.50–56.10) | 16 | 42.57 ±4.72 | 42.9 (34.00–51.00) | 0.773 |
| Albumin [g/l] | 10 | 31.08 ±8.13 | 31.05 (17.20–41.70) | 33 | 30.82 ±6.51 | 31.00 (18.90–47.80) | 16 | 27.21 ±3.49 | 28.9 (19.90–31.00) | 0.136 |
| Serum sodium [mmol/l] | 10 | 138.08 ±3.59 | 139.85 (131.90–141.20) | 33 | 136.77 ±4.73 | 138.40 (120.70–142.10) | 15 | 134.29 ±4.86 | 134.7 (123.20–143.00) | 0.105 |
| Serum potassium [mmol/l] | 10 | 3.82 ±0.52 | 3.77 (2.90–4.44) | 33 | 3.88 ±0.45 | 3.88 (2.73–4.66) | 15 | 3.95 ±0.54 | 3.84 (3.01–4.88) | 0.789 |
| Blood urea nitrogen [mmol/l] | 10 | 6.46 ±3.52 | 5.10 (4.18–15.89) | 33 | 8.03 ±7.05 | 5.58 (2.35–30.40) | 16 | 8.55 ±7.34 | 7.16 (2.24–32.70) | 0.733 |
| Serum creatinine [µmol/l] | 10 | 83.97 ±47.27 | 69.20 (50.29–212.26) | 33 | 91.46 ±96.47 | 65.30 (35.05–463.47) | 16 | 82.96 ±45.59 | 72.75 (32.14–193.66) | 0.926 |
| Child-Pugh score | 10 | 7.80±1.99 | 8.00 (5.00–11.00) | 33 | 8.39 ±2.37 | 8.00 (5.00–13.00) | 15 | 9.47 ±1.73 | 9.00 (8.00–14.00) | 0.141 |
| Child-Pugh class A/B/C, n (%) | 10 | 4 (40.00)/4 (40.00)/2 (20.00) | | 33 | 7 (21.21)/16 (48.49)/10 (30.30) | | 15 | 0 (0.00)/9 (60.00)/6 (40.00) | | 0.161 |
| MELD score | 10 | 10.06 ±3.84 | 8.78 (5.63–18.35) | 33 | 10.33 ±7.95 | 7.66 (–1.42–28.02) | 15 | 12.22 ±5.38 | 12.77 (0.62–20.41) | 0.633 |
| In-hospital death, n (%) | 10 | 0 (0.00) | | 33 | 0 (0.00) | | 16 | 1 (6.25) | | 0.255 |
| Overall death, n (%) | 10 | 1 (10.00) | | 33 | 8 (24.24) | | 16 | 5 (31.25) | | 0.462 |
Discussion
The patients enrolled in our study had several major features. First, our patients formed a group of the northeastern Chinese population with liver cirrhosis. In northeastern China, the most common etiologies of liver cirrhosis are alcohol abuse and hepatitis B virus infection [27]. Indeed, one third of our patients had a history of alcohol abuse and another one third of them had hepatitis B virus infection. Second, a majority of our patients were at the decompensation status, because approximately half of them had developed gastrointestinal bleeding and about two thirds of them had ascites at their admission. Third, most of our patients had relatively severe liver dysfunction, because about two thirds of them were in Child-Pugh class B or C at their admission.
The characteristics of spider nevi and subcutaneous collateral vessels should be interpreted as follows. First, about half of cirrhotic patients had spider nevi of the chest/abdominal wall. Notably, nearly all spider nevi occurred at the chest wall; by comparison, spider nevus was hardly observed at the abdominal wall. Therefore, no comparison was performed according to the location of the spider nevus. Second, about one third of cirrhotic patients had subcutaneous collateral vessels of the chest/abdominal wall. Notably, a majority of them occurred at the abdominal walls.
In line with previous findings by Li et al. [4], our study also confirmed that alcohol abuse was significantly associated with the presence of spider nevus. More importantly, the proportion of subjects with a history of alcohol abuse increased with the number of spider nevi. Considering that alcohol abuse is more common in male patients, we can further explain the association of sex with spider nevus of the chest/abdominal wall. By contrast, the potential etiology of chronic liver diseases did not influence the development of a subcutaneous collateral vessel of the chest/abdominal wall.
Variceal bleeding is the most common type of gastrointestinal bleeding in liver cirrhosis [28–30]. Variceal bleeding is primarily associated with the degree of portosystemic pressure gradient [31], rather than coagulation abnormalities or other abnormal factors [32]. Gastrointestinal endoscopy [31], but not other alternative diagnostic methods [33–35], is the diagnostic gold standard. Our study found that the presence of subcutaneous collateral vessel of the chest/abdominal wall was negatively associated with the probability of previous gastrointestinal bleeding or gastrointestinal bleeding upon admission. Such a significant association might be explained by the fact that subcutaneous collateral vessel of the chest/abdominal wall, a type of spontaneous portosytemic shunt, might produce a lower portosystemic pressure gradient, thereby decreasing the risk of variceal bleeding. However, we must acknowledge that only a proportion of our patients underwent endoscopic examinations for the diagnosis of gastroesophageal varices. Thus, in future, well-designed studies should be performed to explore the association between subcutaneous collateral vessel of the chest/abdominal wall and gastroesophageal varices or hepatic vein pressure gradient. On the other hand, in our study the association of spider nevus with the probability of gastrointestinal bleeding was obscure. A possible explanation is that spider nevus might reflect the severity of liver dysfunction and its associated disturbance of sex hormones, but not that of portal hypertension.
Both spider nevus and subcutaneous collateral vessel of the chest/abdominal wall were positively associated with the severity of liver dysfunction, such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyl transpeptidase, total bilirubin, Child-Pugh score, and MELD score. These findings were similar to those of previous research [4, 5]. To the best of our knowledge, no study has evaluated the impact of spider nevus and subcutaneous collateral vessel of the chest/abdominal wall on the survival of patients with liver cirrhosis. We found that neither of them was associated with in-hospital mortality. However, subcutaneous collateral vessels of the chest/abdominal wall, rather than spider nevus, could significantly predict worse overall survival during the follow-up period. Considering that spider nevus and subcutaneous collateral vessel of the chest/abdominal wall are readily available at the physical examinations, they should be considered as routine markers of liver dysfunction and death in our clinical work.
Our study had limitations. First, numerous studies have shown high prevalence of spider nevus in children regardless of chronic liver diseases [36–38]. Because our patients were adults, rather than children or adolescents, we could not extrapolate from our findings to the pediatric population. Second, spider nevus is distributed all over the body. However, we only collected data regarding spider nevi and subcutaneous collateral vessels on the abdominal and chest walls, and not other body positions. Third, CT examinations might be more accurate and objective for identifying the number of subcutaneous collateral vessels of the chest/abdominal wall [15]. However, we only collected data regarding the presence of subcutaneous collateral vessel of the chest/abdominal wall on the basis of physical examinations, and not CT examinations.
In conclusion, based on the results of a prospective observational study, physical examinations regarding spider nevus and subcutaneous collateral vessel of the chest/abdominal wall should be carefully performed for early and accurate prognostic evaluation of liver cirrhosis.




