Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
RHEUMATOLOGY / STATE OF THE ART PAPER
Investigative biological therapies for primary Sjögren’s syndrome
1
The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Submission date: 2019-07-09
Final revision date: 2019-11-03
Acceptance date: 2019-11-19
Online publication date: 2020-07-15
Publication date: 2024-04-23
Arch Med Sci 2024;20(2):506-516
KEYWORDS
TOPICS
ABSTRACT
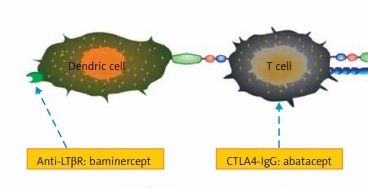
Primary Sjögren’s syndrome (pSS) is a chronic, systemic autoimmune disease characterized by dryness of the eyes and mouth. The histological feature is mononuclear cell infiltration in exocrine glands, primarily salivary and lachrymal glands. As the disease progresses, some other tissues and organs may be involved and extraglandular manifestations ensue. The major current treatments are palliative and empirical, and in most cases the outcomes are not satisfactory. Emerging data indicate a critical role of lymphocytes in its development and progression. While pioneering work targeting B cells has demonstrated some encouraging results, more trials are warranted to validate the safety and efficacy. In addition, modulation of T cell function with abatacept ameliorates the severity of pSS. Furthermore, clinical trials to inhibit important cytokines involved in its formation have been carried out. In this article, we summarize and compare current biological therapies in order to find new and effective treatments for pSS.
REFERENCES (85)
1.
Brito-Zeron P, Theander E, Baldini C, et al. Early diagnosis of primary Sjögren’s syndrome: EULAR-SS task force clinical recommendations. Expert Rev Clin Immunol 2016; 12: 137-56.
2.
Sada PR, Isenberg D, Ciurtin C. Biologic treatment in Sjögren’s syndrome. Rheumatology 2015; 54: 219-30.
3.
Abrol E, Gonzalez-Pulido C, Praena-Fernandez JM, Isenberg DA. A retrospective study of long-term outcomes in 152 patients with primary Sjögren’s syndrome: 25-year experience. Clin Med (Lond) 2014; 14: 157-64.
4.
Gottenberg JE, Seror R, Miceli-Richard C, et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren’s syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS One 2013; 8: e59868.
5.
Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta- analysis. Ann Rheum Dis 2015; 74: 1983-9.
6.
Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol 2014; 6: 247-55.
7.
Saraux A, Pers JO, Devauchelle-Pensec V. Treatment of primary Sjögren syndrome. Nat Rev Rheumatol 2016; 12: 456-71.
8.
Nocturne G, Mariette X. B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol 2018; 14: 133-45.
9.
Sandhya P, Kurien BT, Danda D, Scofield RH. Update on pathogenesis of Sjögren’s syndrome. Curr Rheumatol Rev 2017; 13: 5-22.
10.
Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol 2010; 6: 529-37.
11.
Skopouli FN, Fox PC, Galanopoulou V, Atkinson JC, Jaffe ES, Moutsopoulos HM. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren’s syndrome. J Rheumatol 1991; 18: 210-4.
12.
Verstappen GM, Kroese FGM, Bootsma H. T cells in primary Sjögren’s syndrome: targets for early intervention. Rheumatology (Oxford) 2019; kez004.
13.
Barr JY, Wang X, Kreiger PA, Lieberman SM. Salivarygland-protective regulatory T-cell dysfunction underlies female-specific sialadenitis in the non-obese diabetic mouse model of Sjögren syndrome. Immunology 2018; 155: 225-37.
14.
Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J Autoimmun 2010; 34: 400-7.
15.
Hamza N, Bos NA, Kallenberg CG. B-cell populations and sub-populations in Sjögren’s syndrome. Presse Med 2012; 41: e475-83.
16.
Saadoun D, Terrier B, Bannock J, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum 2013; 65: 1085-96.
17.
Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 2002; 2: 465-75.
18.
Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest 2002; 109: 59-68.
19.
Daridon C, Devauchelle V, Hutin P, et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum 2007; 56: 1134-44.
20.
Mariette X. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis 2003; 62: 168-71.
21.
Verstappen GM, Meiners PM, Corneth OBJ, et al. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren’s syndrome. Arthritis Rheumatol 2017; 69: 1850-61.
22.
Adler S, Korner M, Forger F, Huscher D, Caversaccio MD, Villiger PM. Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjögren’s syndrome: a pilot study. Arthritis Care Res 2013; 65: 1862-8.
23.
Meiners PM, Vissink A, Kroese FG, et al. Abatacept treatment reduces disease activity in early primary Sjögren’s syndrome (open-label proof of concept ASAP study). Ann Rheum Dis 2014; 73: 1393-6.
24.
Haacke EA, van der Vegt B, Meiners PM, et al. Abatacept treatment of patients with primary Sjögren’s syndrome results in a decrease of germinal centres in salivary gland tissue. Clin Exp Rheumatol 2017; 35: 317-20.
25.
Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol 1993; 121: 1121-32.
26.
Morsy DE, Sanyal R, Zaiss AK, Deo R, Muruve DA, Deans JP. Reduced T-dependent humoral immunity in CD20-deficient mice. J Immunol 2013; 191: 3112-8.
27.
Payandeh Z, Bahrami AA, Hoseinpoor R, et al. The applications of anti-CD20 antibodies to treat various B cells disorders. Biomed Pharmacother 2019; 109: 2415-26.
28.
Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjögren’s syndrome: an open-label phase II study. Arthritis Rheum 2005; 52: 2740-50.
29.
Devauchelle-Pensec V, Pennec Y, Morvan J, et al. Improvement of Sjögren’s syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 2007; 57: 310-7.
30.
Seror R, Sordet C, Guillevin L, et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjögren’s syndrome. Ann Rheum Dis 2007; 66: 351-7.
31.
Ramos-Casals M, Garcia-Hernandez FJ, de Ramon E, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol 2010; 28: 468-76.
32.
Devauchelle-Pensec V, Morvan J, Rat AC, et al. Effects of rituximab therapy on quality of life in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol 2011; 29: 6-12.
33.
Dass S, Bowman SJ, Vital EM, et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis 2008; 67: 1541-4.
34.
Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010; 62: 960-8.
35.
Carubbi F, Cipriani P, Marrelli A, et al. Efficacy and safety of rituximab treatment in early primary Sjögren’s syndrome: a prospective, multi-center, follow-up study. Arthritis Res Ther 2013; 15: R172.
36.
Ciccia F, Giardina A, Rizzo A, et al. Rituximab modulates the expression of IL-22 in the salivary glands of patients with primary Sjögren’s syndrome. Ann Rheum Dis 2013; 72: 782-3.
37.
St Clair EW, Levesque MC, Prak ET, et al. Rituximab therapy for primary Sjögren’s syndrome: an open-label clinical trial and mechanistic analysis. Arthritis Rheum 2013; 65: 1097-106.
38.
Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med 2014; 160: 233-42.
39.
Brown S, Navarro Coy N, Pitzalis C, et al. The TRACTISS protocol: a randomised double blind placebo controlled clinical trial of anti-B-cell therapy in patients with primary Sjögren’s syndrome. BMC Musculoskelet Dis 2014; 15: 21.
40.
Bowman SJ, Everett CC, O’Dwyer JL, et al. Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren’s syndrome. Arthritis Rheumatol (Hoboken, NJ) 2017; 69: 1440-50.
41.
Mariette X, Seror R, Quartuccio L, et al. Efficacy and safety of belimumab in primary Sjögren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis 2015; 74: 526-31.
42.
De Vita S, Quartuccio L, Seror R, et al. Efficacy and safety of belimumab given for 12 months in primary Sjögren’s syndrome: the BELISS open-label phase II study. Rheumatology 2015; 54: 2249-56.
43.
Quartuccio L, Salvin S, Corazza L, Gandolfo S, Fabris M, De Vita S. Efficacy of belimumab and targeting of rheumatoid factor-positive B-cell expansion in Sjögren’s syndrome: follow-up after the end of the phase II open-label BELISS study. Clin Exp Rheumatol 2016; 34: 311-4.
44.
Pontarini E, Fabris M, Quartuccio L, et al. Treatment with belimumab restores B cell subsets and their expression of B cell activating factor receptor in patients with primary Sjögren’s syndrome. Rheumatology 2015; 54: 1429-34.
45.
Seror R, Nocturne G, Lazure T, et al. Low numbers of blood and salivary natural killer cells are associated with a better response to belimumab in primary Sjögren’s syndrome: results of the BELISS study. Arthritis Res Ther 2015; 17: 241.
46.
Kadavath S, Bobic S, Efthimiou P. Use of B lymphocyte stimulator inhibitor belimumab may be associated with a decrease in the serum concentration of epidermal growth factor in patients with primary Sjögren’s syndrome. Clin Rheumatol 2015; 34: 1651-2.
47.
Sieger N, Fleischer S, Reiter K, et al. THU0029 epratuzumab influences BCR signalling on TLR9 pre-activated B cells by targeting CD22. Ann Rheum Dis 2014; 72: A174.1-A.
48.
Steinfeld SD, Tant L, Burmester GR, et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren’s syndrome: an open-label phase I/II study. Arthritis Res Ther 2006; 8: R129.
49.
Gottenberg JE, Dorner T, Bootsma H, et al. Efficacy of epratuzumab, an anti-CD22 monoclonal IgG antibody, in systemic lupus erythematosus patients with associated Sjögren’s syndrome: post hoc analyses from the EMBODY Trials. Arthritis Rheum 2018; 70: 763-73.
50.
Dorner T, Posch MG, Li Y, et al. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis 2019; 78: 641-7.
51.
Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors – state of knowledge. Arch Med Sci 2014; 10: 1175-85.
52.
Steinfeld SD, Demols P, Salmon I, Kiss R, Appelboom T. Infliximab in patients with primary Sjögren’s syndrome: a pilot study. Arthritis Rheum 2001; 44: 2371-5.
53.
Steinfeld SD, Demols P, Appelboom T. Infliximab in primary Sjögren’s syndrome: one-year followup. Arthritis Rheum 2002; 46: 3301-3.
54.
Mariette X, Ravaud P, Steinfeld S, et al. Inefficacy of infliximab in primary Sjögren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis Rheum 2004; 50: 1270-6.
55.
Zandbelt MM, de Wilde P, van Damme P, Hoyng CB, van de Putte L, den Hoogen F. Etanercept in the treatment of patients with primary Sjögren’s syndrome: a pilot study. J Rheumatol 2004; 31: 96-101.
56.
Sankar V, Brennan MT, Kok MR, et al. Etanercept in Sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum 2004; 50: 2240-5.
57.
Moutsopoulos NM, Katsifis GE, Angelov N, et al. Lack of efficacy of etanercept in Sjögren syndrome correlates with failed suppression of tumour necrosis factor alpha and systemic immune activation. Ann Rheum Dis 2008; 67: 1437-43.
58.
Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol 2017; 13: 217-33.
59.
Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 2004; 202: 49-66.
60.
Shen L, Suresh L, Wu J, et al. A role for lymphotoxin in primary Sjögren’s disease. J Immunol 2010; 185: 6355-63.
61.
Fava RA, Kennedy SM, Wood SG, et al. Lymphotoxin-beta receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of Sjögren’s syndrome. Arthritis Res Ther 2011; 13: R182.
62.
Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad AI. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjögren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther 2009; 11: R24.
63.
St Clair EW, Baer AN, Wei C, et al. Clinical efficacy and safety of baminercept, a lymphotoxin beta receptor fusion protein, in primary Sjögren’s syndrome: results from a phase ii randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2018; 70: 1470-80.
64.
Roescher N, Tak PP, Illei GG. Cytokines in Sjögren’s syndrome: potential therapeutic targets. Ann Rheum Dis 2010; 69: 945-8.
65.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46-56.
66.
Norheim KB, Harboe E, Goransson LG, Omdal R. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome – a double blind, randomised clinical trial. PLoS One 2012; 7: e30123.
67.
Roescher N, Tak PP, Illei GG. Cytokines in Sjögren’s syndrome. Oral Dis 2009; 15: 519-26.
68.
Justet A, Ottaviani S, Dieude P, Taille C. Tocilizumab for refractory organising pneumonia associated with Sjögren’s disease. BMJ Case Rep 2015; 2015: bcr2014209076.
69.
Marino A, Narula S, Lerman MA. First pediatric patient with neuromyelitis optica and Sjögren syndrome successfully treated with tocilizumab. Pediatr Neurol 2017; 73: e5-e6.
70.
Komai T, Shoda H, Yamaguchi K, et al. Neuromyelitis optica spectrum disorder complicated with Sjögren syndrome successfully treated with tocilizumab: a case report. Mod Rheumatol 2016; 26: 294-6.
71.
Jakez-Ocampo J, Atisha-Fregoso Y, Llorente L. Refractory primary Sjögren syndrome successfully treated with bortezomib. J Clin Rheumatol 2015; 21: 31-2.
72.
Sun R, Gu W, Ma Y, Wang J, Wu M. Relapsed/refractory acquired thrombotic thrombocytopenic purpura in a patient with Sjögren syndrome: case report and review of the literature. Medicine (Baltimore) 2018; 97: e12989.
73.
Kamal A, Khamashta M. The efficacy of novel B cell biologics as the future of SLE treatment: a review. Autoimmun Rev 2014; 13: 1094-101.
74.
De Vita S, Quartuccio L, Salvin S, et al. Sequential therapy with belimumab followed by rituximab in Sjögren’s syndrome associated with B-cell lymphoproliferation and overexpression of BAFF: evidence for long-term efficacy. Clin Exp Rheumatol 2014; 32: 490-4.
75.
Jiang S, Wang Z, Ouyang H, Liu Z, Li L, Shi Y. Aberrant expression of cytokine interleukin 9 along with interleukin 4 and interferon gamma in connective tissue disease-associated interstitial lung disease: association with severity of pulmonary fibrosis. Arch Med Sci 2016; 12: 101-6.
76.
Danczak-Pazdrowska A, Kowalczyk M, Szramka-Pawlak B, et al. Interleukin-17A and interleukin-23 in morphea. Arch Med Sci 2012; 8: 1089-95.
77.
Schurich A, Raine C, Morris V, Ciurtin C. The role of IL-12/23 in T cell-related chronic inflammation: implications of immunodeficiency and therapeutic blockade. Rheumatology 2018; 57: 246-54.
78.
Guo J, Gu M, Zhang W, Liu Y, Qian C, Deng A. Aberrant IL-35 levels in patients with primary Sjögren’s syndrome. Scand J Immunol 2018; 88: e12718.
79.
Yu X. Autoantibodies against muscarinic acetylcholine receptor M sub 3 sub in Sjögren rsquo s syndrome and corresponding mouse models. Front Biosci 2018; 23: 2053-64.
80.
Nayar S, Campos J, Smith CG, et al. Phosphatidylinositol 3-kinase delta pathway: a novel therapeutic target for Sjögren’s syndrome. Ann Rheum Dis 2019; 78: 249-60.
81.
Pollard RP, Abdulahad WH, Vissink A, et al. Serum levels of BAFF, but not APRIL, are increased after rituximab treatment in patients with primary Sjögren’s syndrome: data from a placebo-controlled clinical trial. Ann Rheum Dis 2013; 72: 146-8.
82.
Karmacharya P, Poudel DR, Pathak R, et al. Rituximab-induced serum sickness: a systematic review. Semin Arthritis Rheum 2015; 45: 334-40.
83.
Moerman RV, Arends S, Meiners PM, et al. EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) is sensitive to show efficacy of rituximab treatment in a randomised controlled trial. Ann Rheum Dis 2014; 73: 472-4.
84.
Cornec D, Devauchelle-Pensec V, Mariette X, et al. Severe health-related quality of life impairment in active primary Sjögren’s syndrome and patient-reported outcomes: data from a large therapeutic trial. Arthritis Care Res 2017; 69: 528-35.
85.
Cornec D, Devauchelle-Pensec V, Mariette X, et al. Development of the Sjögren’s Syndrome Responder Index, a data-driven composite endpoint for assessing treatment efficacy. Rheumatology 2015; 54: 1699-708.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.