Introduction
Postmenopausal osteoporosis (PMO) is a global healthcare problem that affects approximately 40% of postmenopausal women worldwide [1]. Estrogen deficiency is believed to be the primary cause of PMO, which is attributed to a disequilibrium between osteoblast-mediated bone formation and osteoclast-mediated bone resorption and eventually results in osteoporotic fracture [2–5]. At present, there are no ideal therapeutic drugs for the treatment of PMO. For example, hormone replacement therapy (HTR) has been widely used in the prevention of PMO by counteracting the loss of estrogen in postmenopausal women, while emerging side effects, such as increasing the risk of endometrial cancer, cardiovascular diseases and atypical fractures, limit its clinical applications [3, 6]. There is mounting evidence that traditional Chinese herbal medicines and their bioactive components, including Fructus ligustri lucidi [7], Sambucus williamsii [8], Epimedium [9] and Polygonum multiflorum [10], exert beneficial activity against ovariectomy (OVX)-induced osteoporosis. However, the precise mechanisms regarding the anti-osteoporotic activity of traditional herbal medicines have not been completely clarified.
Schisandrin B (SchB) is the main bioactive ingredient derived from Schisandra chinensis, and it performs a variety of biological functions, including antioxidant, anti-inflammatory and anti-DNA oxidative damage activities [11–13]. It has also been reported that SchB prevents cartilage degeneration in a rat osteoarthritis model [14]. Intriguingly, active fractions from Schisandra chinensis stimulate osteoblast proliferation and increase alkaline phosphatase activity in vitro, suggesting that they may possess potential activity against osteoporosis [15]. However, the underlying molecular mechanisms and the effects of SchB on OVX-induced osteoporosis have not been reported in an experimental rat model of estrogen deficiency.
In the present study, we report for the first time that SchB alleviated OVX-induced osteoporosis by activating the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. In osteoporotic rats, impairment of PI3K/AKT signaling is associated with bone loss [16, 17]. In vitro experiments also show that upregulation of PI3K/AKT activity facilitates osteoblast differentiation and increases osteoblast-specific gene expression [18, 19]. Overactivation of PI3K/AKT signaling can also modulate expression of β-catenin protein, which is a vital component in the wnt/β-catenin pathway and participates in osteogenesis [20]. We investigated the osteoprotective effects of SchB, and the results demonstrated that SchB could prevent Ca excretion and bone loss in an ovariectomized rat model. The underlying mechanism was mediated, at least partially, through activation of PI3K/AKT signaling in the bone. These findings suggested that SchB might serve as a potential therapeutic agent for the treatment of osteoporosis.
Material and methods
Animal treatment
Two-month-old female Sprague-Dawley rats (n = 60; body weight: 200 ±20 g) were purchased from Slac Laboratory Animal, Shanghai, China and allowed to acclimate to the environment for 1 week. After 1 week of sham or OVX operation, the rats in the sham-operated (Sham) group and OVX group received sodium carboxymethylcellulose (CMC) treatment with intragastric administration; the rats in the estradiol valerate (EV) group underwent OVX operation combined with EV treatment (1 mg/kg/day), EV (1 mg/ml) was dissolved in 0.5% CMC with intragastric administration; the rats in the SchB-L group underwent OVX operation combined with low-dosage SchB (SchB-L; 10 mg/kg/day) treatment, SchB was dissolved in 0.5% CMC (10 mg/ml CMC) with intragastric administration; the rats in the SchB-H group underwent OVX operation combined with high-dosage SchB (SchB-H; 50 mg/kg/day) treatment, SchB was dissolved in 0.5% CMC (50 mg/ml CMC) with intragastric administration. Twelve rats were assigned to each group (n = 12 in each group). EV was obtained from Elpharm Lille SAS (Paris, France) and SchB was obtained from Shanghai PureOne Biotechnology (Shanghai, China). After 8 weeks of treatment, the rats were killed by an overdose of sodium pentobarbital (2%; 200 mg/kg; Sigma-Aldrich, Merck KGaA, Germany) 24 h after their last treatment. Serum and tibias were immediately collected and stored at –80°C for further analysis. In addition, tibias were fixed with 4% formalin at room temperature and embedded in paraffin for histomorphological analysis. The experiment was approved by the Ethics Committee of Affiliated Hospital of Yanbian University (Yanji, China) and was performed in accordance with its guidelines.
Biochemical markers in serum and urine
The levels of calcium (Ca), phosphorus (P) and creatinine (Cre) in serum and urine were measured by commercial kits (Nanjing Jiancheng Biology Engineering Institute, Nanjing, China) according to the manufacturer’s protocol. Alkaline phosphatase (ALP; Elabscience Biotechnology Co., Ltd. Wuhan, China), osteocalcin (Immutopics, Inc., San Clemente, CA, USA), tartrate-resistant acid phosphatase-5b (TRAP-5b; Immunodiagnostic Systems, Scottsdale, AZ, USA), and C-terminal telopeptide of type 1 collagen (CTX; Elabscience Biotechnology Co., Ltd. Wuhan, China) were detected by rat bioactive ELISA kits according to each manufacturer’s protocol. Serum intact parathyroid hormone (PTH) and osteocalcin were measured using chemiluminescence immunoassays (Roche Diagnostics, Mannheim, Germany). Serum 25(OH) vitamin D3 assay was performed using a DiaSorin kit on a LIAISON automated immunoassay analyzer (DiaSorin, Saluggia, Italy).
Bone calcium content and bone mineral density (BMD)
To measure Ca content in the tibia, the tibia was incinerated using a muffle furnace (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 800°C for 12 h, and then 10 mg of bone ash was dissolved in 1 ml of 37% HCl diluted with Milli-Q water. The calcium content was determined using a commercial kit (Nanjing Jiancheng Biology Engineering Institute) according to the manufacturer’s protocol. The BMD of the femur and tibia was measured by dual-energy X-ray absorptiometry (DEXA) (Lunar DPXIQ, GE Healthcare, USA).
Histomorphology and TRAP staining
Tibias were collected immediately following sacrifice and fixed with 4% formalin at room temperature for 24 h, then decalcified in 0.5 M EDTA (pH = 8.0) and embedded in paraffin. Sections of 5 µm were cut and stained with hematoxylin and eosin (H&E; Beyotime Institute of Biotechnology, Haimen, China) and safranin O (Nanjing SenBeiJia Biological Technology Co., Ltd., Nanjing, China) and visualized under a microscope (Leica DM 2500).
TRAP staining was used for the identification of osteoclasts according to the manufacturer’s instructions (Sigma-Aldrich). The number of osteoclasts was quantified below the growth plates in the proximal tibial metaphysis and visualized under a microscope (Leica DM 2500).
Western blotting
Proteins were extracted with radio immunoprecipitation assay (RIPA) buffer (Beyotime Institute of Biotechnology, Haimen, China). Western blotting assays were performed as previously described [21]. After blocking with 5% skim milk, the membrane was incubated with primary antibodies as follows: anti-PI3K (dilution: 1 : 1,000; Cell Signaling Technology, USA), anti-p-PI3K (dilution: 1 : 1,000; Cell Signaling Technology, USA), anti-Akt (dilution: 1 : 500; Santa Cruz Biotechnology), anti-p-Akt (dilution: 1 : 500; Santa Cruz Biotechnology) and anti-β-catenin (dilution: 1 : 500; Santa Cruz Biotechnology). After incubation with primary antibodies, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (cat. no. sc-516102; dilution: 1 : 10,000; Santa Cruz Biotechnology), followed by visualization using chemiluminescence (Thermo Fisher Scientific, Inc.). β-actin (cat. no. sc-130065; 1 : 2,000; Santa Cruz Biotechnology) was used as the loading control antibody. Signals were analyzed with Quantity One software version 4.5 (Bio-Rad).
Ethics approval
The experiment was approved by the Ethics Committee of Affiliated Hospital of Yanbian University (Yanji, China) and was performed in accordance with its guidelines.
Statistical analysis
The data from these experiments were reported as the mean ± standard error for each group. All statistical analyses were performed using Prism version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Student’s t-test was used to analyze the differences between two groups. Intergroup differences were analyzed by one-way analysis of variance, followed by a post hoc Tukey test for multiple comparisons. P < 0.05 was considered to indicate a statistically significant difference.
Results
Effect of SchB on body weight and uterus index
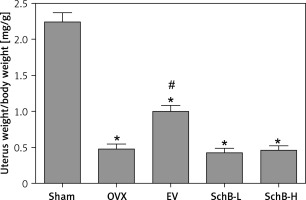
Increased body weight and lipid metabolism disorders are usually accompanied by estrogen deficiency in the experimental rodent model [22]. Consistent with previous results, our findings also demonstrated that body weight was significantly increased in OVX-operated rats compared with that of rats in the sham-operated group during the experimental period, while EV administration significantly reversed the OVX-induced increase in body weight in rats. However, both low and high concentrations of SchB had no obvious effect on body weight in OVX rats (Table I). As expected, atrophy of the uterus was observed in OVX rats, while EV treatment markedly accelerated the growth of the uterus in OVX rats. There was no significant improvement in the uterus index in OVX rats treated with SchB (Figure 1).
Table I
Body weight of rats was recorded every 2 weeks during the experimental period
| Week | Sham | OVX | EV | SchB-L | SchB-H |
|---|---|---|---|---|---|
| 0 | 205.6 ±7.8 | 209.3 ±8.2 | 212.4 ±7.6 | 208.7 ±6.9 | 211.4 ±7.5 |
| 2 | 214.4 ±9.2 | 235.8 ±10.1 | 224.3 ±9.1 | 231.2 ±9.3 | 234.5 ±8.2 |
| 4 | 233.5 ±10.7 | 256.5 ±12.6* | 231.3 ±12.1# | 258.4 ±11.5 | 269.1 ±14.6 |
| 6 | 246.1 ±12.4 | 283.4 ±12.5** | 253.1 ±13.6 ## | 279.6 ±11.6 | 269.1 ±12.5 |
| 8 | 258.3 ±14.2 | 311.2 ±15.2*** | 272.4 ±13.6### | 304.8 ±13.7 | 298.4 ±14.2 |
Effect of SchB on Ca metabolism and BMD
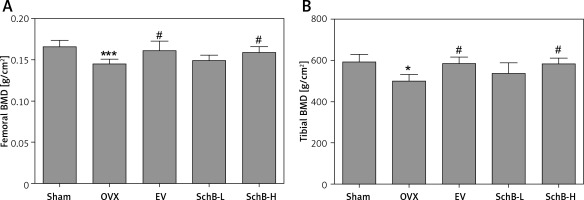
Ca is the most important mineral component in the skeletal system, and Ca intake can affect bone health by increasing BMD [23]. Estrogen deficiency in animal models induces osteoclastogenesis and bone resorption, which accelerate Ca loss by urinary excretion and release of skeletal Ca into the blood [24]. Our results demonstrated that serum Ca, serum P and tibial Ca were significantly decreased in OVX rats compared with those of rats in the sham-operated group. In contrast, urinary Ca was dramatically increased in OVX rats, suggesting that Ca loss was observed in estrogen-deficient rats. Interestingly, high-dosage SchB treatment alleviated OVX-induced Ca loss but had no effect on urinary P levels (Table II). To investigate whether SchB improved Ca homeostasis by regulating calciotropic hormone levels, three mainly calciotropic hormones – 25(OH)VD3, PTH and calcitonin – were measured in the serum. The results indicated that 25(OH)VD3 and calcitonin were significantly decreased and PTH was significantly elevated in OVX rats compared to rats in the sham-operated group. Conversely, the OVX rats treated with SchB at a high dosage showed an increase in serum 25(OH)VD3 and calcitonin and a decrease in serum PTH (Figures 2 A–C), reflecting that SchB might maintain Ca homeostasis in OVX rats by regulating calciotropic hormones. Furthermore, we explored the effect of SchB on femoral and tibial BMD in OVX rats. As shown in Figures 3 A and B, compared with sham-operated rats, the BMD of the femur and tibia significantly declined from 0.166 ±0.008 and 0.148 ±0.010 to 0.145 ±0.006 and 0.125 ±0.009, respectively, in OVX rats 8 weeks after the surgical operation. However, the BMD of the femur and tibia in the OVX rats was increased and reached 0.158 ±0.007 and 0.146 ±0.007, respectively, after high-dosage SchB treatment.
Table II
Biochemical parameters in OVX mice treated with SchB
| Parameter | Sham | OVX | EV | SchB-L | SchB-H |
|---|---|---|---|---|---|
| Serum Ca [mg/dl] | 10.21 ±0.28 | 9.05 ±0.30* | 10.35 ±0.36# | 9.35 ±0.35 | 10.06 ±0.37# |
| Serum P [mg/dl] | 5.79 ±0.34 | 5.04 ±0.30* | 5.94 ±0.41# | 5.17 ±0.31 | 5.83 ±0.35# |
| Urinary Ca/Cre [mg/mg] | 0.167 ±0.018 | 0.331 ±0.035* | 0.194 ±0.028# | 0.301 ±0.037 | 0.213 ±0.026# |
| Urinary P/Cre [mg/mg] | 1.22 ±0.15 | 1.31 ±0.26 | 1.35 ±0.38 | 1.23 ±0.37 | 1.18 ±0.39 |
| Tibial Ca/ash [mg/mg] | 0.452 ±0.008 | 0.402 ±0.009* | 0.467 ±0.011# | 0.408 ±0.012 | 0.459 ±0.015# |
| Tibial Ca [mg/g] | 167.5 ±10.44 | 146.8 ±14.8* | 169.6 ±12.33# | 142.78 ±14.2 | 159.6 ±13.56# |
Figure 2
Effect of SchB on the level of calciotropic hormones in serum. After treatment with SchB at low or high dosage for 8 weeks, the levels of 25-hydroxy vitamin D3 [25(OH)VD3] (A), parathyroid hormone (PTH) (B) and calcitonin (C) were measured by ELISA assays. N = 12 in each group
*P < 0.05, ***p < 0.001 vs. sham-operated group; #p < 0.05, ###p < 0.001 vs. OVX-operated group.
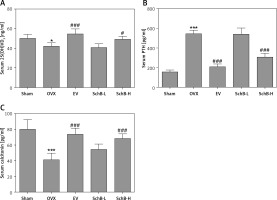
Figure 3
Effect of SchB on BMD of the femur and tibia. After treatment with SchB at low or high dosage for 8 weeks, the BMD of femur (A) and tibia (B) was measured by dual-energy X-ray absorptiometry. N = 12 in each group
*P < 0.05, ***p < 0.001 vs. sham-operated group; #p < 0.05 vs. OVX-operated group.
Effect of SchB on bone formation and bone resorption markers
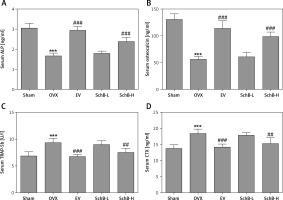
The effects of SchB on the levels of bone formation markers, ALP and osteocalcin, and bone resorption markers, TRAP-5b and CTX, are shown in Figure 4. At 8 weeks after OVX surgical operation, the levels of ALP (Figure 4 A) and osteocalcin (Figure 4 B) in the serum were significantly decreased by 45.8% and 57.1%, respectively, and serum TRAP-5b (Figure 4 C) and CTX (Figure 4 D) were markedly elevated by 37.3% and 33.2%, respectively, in the OVX-operated group compared with the sham-operated group. SchB at a high dosage had a significant effect on increasing the activity of ALP by 44.0% and osteocalcin by 76.9% and suppressing the activity of TRAP-5b by 19.6% and CTX by 17.0%, compared to the OVX-operated group.
Figure 4
Effect of SchB on bone formation and bone resorption markers. After treatment with SchB at low or high dosage for 8 weeks, the serum levels of alkaline phosphatase (ALP; A) osteocalcin (B); tartrate-resistant acid phosphatase 5b (TRAP-5b, C); C-terminal telopeptide of type 1 collagen (CXT, D) were measured by ELISA assays. N = 12 in each group
***p < 0.001 vs. sham-operated group, ##p < 0.01, ###p < 0.001 vs. OVX-operated group.
Effect of SchB on metabolism of trabecular bone and growth plate cartilage in the tibia
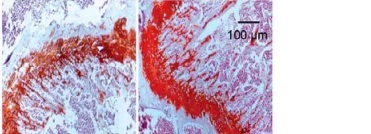
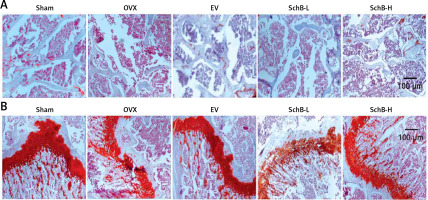
To determine the effects of SchB on trabecular bone and cartilage metabolism, TRAP, H&E and safranin O staining were performed in the tibia of OVX-operated rats with or without SchB treatment. TRAP staining showed that mature osteoclast numbers were significantly increased in the proximal metaphysis of tibia from OVX-operated rats compared to the sham-operated group. However, treatment with SchB blocked osteoclastogenesis in the tibia of OVX-operated rats (Figures 5 A, B). In addition, H&E staining was performed to assess the trabecular bone structure in the proximal metaphysis of the tibia, and we observed a notable reduction in cancellous bone mass and network connectivity in OVX-operated rats, while high-dosage SchB treatment dramatically reversed the OVX-induced bone loss (Figure 6 A). Furthermore, safranin O staining revealed that the degradation of epiphyseal plate cartilage was detected in the proximal tibia of OVX-operated rats, suggesting that cartilage formation was delayed in OVX-operated rats. Intriguingly, high-dosage SchB administration protected against OVX-induced cartilage degradation (Figure 6 B).
Figure 5
Effect of SchB on osteoclast differentiation. After treatment with SchB at low or high dosage for 8 weeks, tartrate-resistant acid phosphatase (TRAP) staining was used to evaluate osteoclastogenesis (A and B; magnification 50.; scale bar = 100 μm). N = 12 in each group
*P < 0.05 vs. sham-operated group; #P < 0.05 vs. OVXoperated group. Red arrows indicate mature osteoclast.
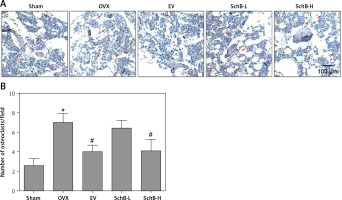
Figure 6
Effect SchB on metabolism of trabecular bone and growth plate cartilage. Hematoxylin and eosin (H&E; A; magnification 50.; scale bar = 100 μm) and safranin O staining (B; magnification 50.; scale bar = 100 μm) were performed to analyze trabecular bone and cartilage degeneration, respectively
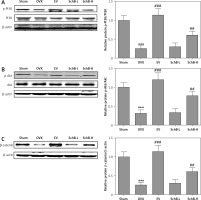
SchB activates PI3K/Akt signaling in the bone of OVX rats
Previous studies have indicated that impaired PI3K/Akt signaling was observed in the liver, skeletal muscle and tibia in an OVX-operated animal model [17, 25]. Our results also revealed that the protein expression of phosphorylated PI3K and Akt was inhibited in the proximal tibia of OVX-operated rats compared with that of rats in the sham-operated group. SchB treatment at high dosage markedly reversed the OVX-induced suppression of phosphorylated PI3K and Akt (Figure 7 A, B). β-catenin is a vital component of the wnt/β-catenin pathway and plays an important role in osteogenesis [26]. As expected, the protein expression of β-catenin was significantly decreased in the proximal tibia of OVX-operated rats compared with the sham group. High-dosage SchB treatment increased β-catenin protein expression in OVX rats (Figure 7 C).
Figure 7
SchB activated PI3K/Akt/β-catenin signaling in bone of OVX rat. After treatment with SchB at low or high dosage for 8 weeks, protein expression of PI3K and p-PI3K (A), Akt and p-Akt (B), and β-catenin (C) was measured by western blotting. N = 12 in each group
***P < 0.001 vs. sham-operated group; ##p < 0.01, ###p < 0.001 vs. OVX-operated group.
Discussion
The OVX rat, as an animal model of estrogen deficiency, has been widely used for mimicking PMO in women [27]. OVX-induced decline in ovarian estrogen production leads to a rapid loss of trabecular bone mass, which eventually results in an increased risk of fragility fracture [27]. Estrogens play a crucial role in bone metabolism, but it is still difficult to expound the precise mechanisms by which estrogens modulate Ca metabolism and bone homeostasis. Notably, the canonical osteoprotegerin (OPG)/receptor activator of the nuclear factor κB ligand (RANKL)/RANK axis has been proposed as a key signaling pathway in mediating the molecular association between estrogen deficiency and bone deterioration [28]. In addition, reactive oxygen species and inflammatory responses potentiate bone degenerative processes in the presence of estrogen deficiency [29, 30]. In the present study, we aimed to determine whether SchB could attenuate bone deterioration caused by estrogen deficiency by regulating the PI3K/Akt pathway in an experimental rat model.
A previous study indicated that the PI3K/Akt pathway is a crucial factor for bone formation [31]. Inhibition of p-AKT in vivo and in vitro is accompanied by glucocorticoid-induced osteoporosis and osteoblast apoptosis [32, 33]. Xi et al. showed that the protein expression of phospho-PI3K and phospho-Akt is dramatically decreased in bone tissue from osteoporotic rats, and the PI3K/Akt pathway may be involved in the inhibition of osteoporosis through acceleration of osteoblast proliferation and bone formation [16]. In OVX mice [25, 34], activation of the PI3K/Akt pathway can promote bone formation and prevent bone resorption. In accordance with previous studies, the current results showed that OVX caused downregulation of phospho-PI3K and phospho-Akt in the proximal tibia. More importantly, SchB treatment showed a significant increase in phospho-PI3K and phospho-Akt in the proximal tibia of OVX-operated rats. We also found that estrogen withdrawal-induced calcium metabolism disequilibrium, trabecular bone loss, osteoclastogenesis and epiphyseal plate cartilage degradation were blocked by treatment with SchB, and the underlying mechanism was mediated, at least partially, through activation of the PI3K/Akt pathway in the local bone.
Our findings also revealed that SchB functioned as an activator of β-catenin protein expression in the proximal tibia of OVX-operated rats. Activation of Wnt signaling by inhibiting the activity of glycogen synthase kinase-3β reduces the phosphorylation of β-catenin, which results in increased β-catenin in the cytosol and its translocation into the nucleus. Subsequently, the interaction of β-catenin with T cell factor/lymphoid enhancer binding factor enhances gene transcription to trigger osteoblast proliferation [26]. In vivo and in vitro studies have confirmed that β-catenin is a critical regulator of chondrocyte and osteoblast differentiation, the generation of ossification centers and perichondrial bone formation during the process of cartilage and bone development [33, 35]. In a previous study, icariin protected against glucocorticoid-induced osteoporosis by increasing β-catenin expression [33]. Moreover, improved bone mass and strength are correlated with activated Wnt/β-catenin signaling in OVX mice [36]. Our results indicated that SchB could inhibit OVX-induced osteoporosis by enhancing β-catenin signaling. Interestingly, as a downstream target, β-catenin can be activated by phospho-PI3K and phospho-Akt, reflecting that the PI3K/Akt/β-catenin signaling cascade is involved in osteogenic proliferation and differentiation and inhibits the development of osteoporosis [20, 33]. Our findings also provided a possible mechanism by which PI3K/Akt/β-catenin signaling might participate in SchB-facilitated anti-osteoporotic activity in OVX-operated rats.
Usually, the majority of estrogen-like hormones, phytoestrogens and active components of Chinese traditional herbs are used as accessory therapeutic medicines for alleviating estrogen-related side effects in postmenopausal women undergoing anti-osteoporotic therapy [37]. Theoretically, the anti-osteoporotic activity of estradiol or estradiol valerate is obviously better than phytoestrogens or active pharmaceutical ingredients at equivalent dosage [38]. For example, subcutaneous administrations of genistein (10 mg/kg/day) shows similar effectiveness of 17β-estradiol (2 µg/kg/day) in bone protection [38]. Soy isoflavone (60 mg/kg/day) has positive effects on bone metabolism in OVX rats; the effective concentration of soy isoflavone is significantly higher than 17β-estradiol in OVX mice [39]. Puerarin (≥ 2 mg/kg/day), a major isoflavonoid isolated from Puerariae radix, prevents bone loss in OVX mice, which is consistent with 17β-estradiol (0.03 µg/day) [40]. In the present study, our results demonstrated that high-dosage SchB treatment (50 mg/kg/day) had an equivalent anti-osteoporotic effect of EV treatment (1 mg/kg/day) in OVX rats.
Abundant Ca supplementation, at least 1000–1200 mg daily, has long been recommended for the prevention and treatment of osteoporosis [23]. Unfortunately, some side effects have been observed, including cardiovascular events, kidney stones and acute gastrointestinal symptoms [41–43]. Therefore, there has been a growing number of publications focusing on the natural regulator of Ca metabolism. For example, ursolic acid, icariin and quercetin effectively improve calcium metabolism and bone mineral density [33, 44, 45]. Our results showed that SchB had a positive effect on Ca balance by modulating the activity of calciotropic hormones, including 25(OH)VD3, PTH and calcitonin.
However, there are some limitations to our study. The quantitative biological parameters of trabecular bone were not measured by micro-CT analysis. Moreover, the underlying molecular mechanisms of the effect of SchB on Ca metabolism are still not clear in our study.
In conclusion, our results demonstrated that SchB exerted anti-osteoporotic activity in OVX-operated rats, as shown by the improvement of Ca homeostasis and trabecular bone and cartilage metabolism, and the inhibition of osteoclast activity. The studies of the underlying molecular mechanism revealed that SchB accelerated the phosphorylation of PI3K and Akt and subsequently up-regulated the expression of β-catenin in OVX-operated rats, suggesting that the activated PI3K/Akt/β-catenin signaling pathway might have a beneficial effect on OVX-induced osteoporosis.