Introduction
Knee osteoarthritis (KOA) is a common disorder characterized by cartilage deterioration and joint space shortening [1]. Patients commonly get a variety of therapies to halt or stop the progression of KOA; however, no medication has been proven to do so. The present treatment focuses primarily on symptom remission, with the prime objective of pain relief and function improvement. Nonsurgical illnesses are treated with both nonpharmacological and pharmacological therapy [2]. Non-pharmacological therapy such as diet and exercise are frequently recommended; however, they are not always followed [3]. The most prevalent pharmacological therapy for KOA is oral glucosamine, chondroitin, acetaminophen, celecoxib, and chondroitin. However, non-steroid anti-inflammatory drugs (NSAIDs) and analgesics are usually associated with side effects [4]. Total knee arthroplasty can be one of the treatment options, but it has a complicated surgical procedure. The American College of Rheumatology (ACR) authorized intra-articular hyaluronic acid (HA) injections in the therapy of patients with KOA in 2012 [5], but owing to its low effectivity, various studies [6–10] highly recommend the use of platelet-rich plasma (PRP). PRP is an autologous product made from a patient’s blood using a gradient density centrifugation procedure. PRP contains a variety of growth factors and other bioactive compounds that have been shown to help control abnormal inflammatory processes, rebuild tissue structures, and promote tissue repair. Autologous PRP has a low risk of immunological responses and infectious disease transmission, and it has been routinely utilized to treat rotator cuff tendinopathy. For example, Lin et al. found that intra-articular injections of leukocyte-poor PRP can give a clinically meaningful functional improvement in patients with mild-to-moderate osteoarthritis of the knee for at least 1 year. However, some studies also reported less efficacy and limited use of PRP.
Aim
Therefore, the present meta-analysis aimed to systematically analyze the different randomized controlled trials (RCTs) comparing the effectiveness of HA vs. PRP for the treatment of knee osteoarthritis and see how effective and safe intra-articular PRP is for KOA patients.
Material and methods
Search strategy
This meta-analysis is based on an extensive literature search conducted using Medline (PubMed), Cinahl (Ebsco), Scopus, Web of Sciences, and Cochrane Register of Controlled Trials (CENTRAL) databases from the year 2000 to 2021. The following search words were used: knee osteoarthritis, HA, PRP, meta-analysis, and RCTs.
Study selection or inclusion/exclusion criteria
Studies were selected randomly irrespective of their language, publication status, or study type, and those having potentially relevant titles and abstracts were scanned, and their full-text versions were read. Included RCTs [6–19] with sufficient event data were selected as per the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (registration number CH#/IRB/2021/555). Studies with insufficient data, non-randomized studies, quasi-experimental studies, retrospective and cohort studies, and related studies published before 2000 were excluded.
Data extraction and quality assessment
After identifying relevant articles that met the desired inclusion criteria, event data were extracted using a predefined data extraction form, and the demographic summary is presented in Table I. It includes the following items: author of the study, publication year, study type, duration of the study, and number of patients included, their age, sex ratio, and dose of drugs used for both intervention (PRP) treated patients and control (HA) treated patients, a parameter to assess positive outcome and statistical significance of results in terms of the p-value. In order to assess the methodological quality of the included studies, the Cochrane Collaboration’s risk of bias tool was used, including the criteria of randomization, allocation concealment, blinding, and completeness of follow-up as the significant assessment parameters. The risk of bias for each item was graded as high, low, or unclear risk.
Table I
Demographic summary of the included studies
Sources of heterogeneity
The investigated heterogeneity sources were full-text publications versus abstracts, randomized controlled trials of patients of various age groups, different numbers of patients, variable duration of treatment, different scales of analysis, and comparison of PRP with different control medicines.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine (CH#/IRB/2021/555) and informed consent was not required.
Quantitative data synthesis
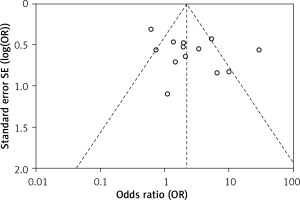
In order to assess the comparative efficiency of HA and PRP in the treatment of knee osteoarthritis, patients of different age groups were treated with either HA or PRP. Their positive outcomes were reported in terms of either MRI findings, EuroQol visual analog scale (EQ-VAS), International Knee Documentation Committee (IKDC) subjective scores, Knee Injury and Osteoarthritis Outcome Score (KOOS), or Western Ontario and McMaster Universities Arthritis Index (WOMAC) score. Meta-analysis was performed using this extracted data, and statistical parameters such as diagnostic odds ratios and relative risk with a 95% confidence interval were calculated by the Mantel-Haenszel method with random bivariate effects using RevMan software (Review Manager, RevMan, Version 5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2020) along with their respective forest plots. Meta-analyses were done using a random-effects model (Mantel-Haenszel method), and heterogeneity in the included studies was evaluated using the τ2 value, χ2 value, I2 value, and Z value. A p-value < 0.00001 was considered statistically significant. Publication bias of the included studies was summarized in Table II and assessed via funnel plot in which the log risk ratio of each study was plotted against its standard error.
Table II
Risk assessment for included studies
Results
Literature search results
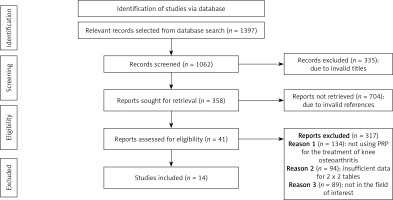
We found a total of 1397 studies through electronic scans from different databases. We excluded 335 studies by reading their titles and abstracts, and 1062 records were screened among these studies. Further, due to invalid references and duplicity, we excluded 704 studies and included only 358 studies for final screening. Out of these 358 studies, 317 were excluded based on the inclusion criteria, and the eligibility of the remaining 41 studies was assessed further. The critical reasons for omission were inadequate evidence and inappropriate comparison criteria to create 2x2 tables for review. Finally, for the meta-analysis, 14 studies that fulfill the inclusion criteria, i.e., use of HA vs. PRP, were used (Figure 1).
Bias assessment
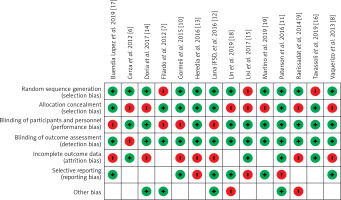
The outcome of risk of bias evaluation via RevMan software is shown in Figure 2. Overall, there was a moderate to high risk of bias due to risk related to randomization, blinding, and selective reporting domains. As shown in Figure 3, the funnel plot was symmetrical and indicated a low possibility of publication bias.
Meta-analysis results
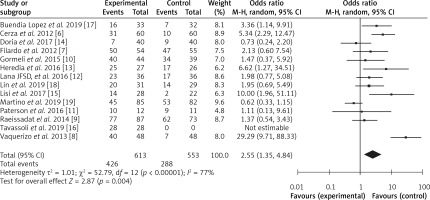
The diagnostic odds ratio was calculated using RevMan software, and a forest plot was constructed, as shown in Figure 4. We obtained the pooled odds ratio (OR) value of 2.55 with 95% CI ranging from 1.35 to 4.84. Data were heterogeneous with a τ2 value of 1.01, χ2 value of 52.79, I2 value of 77%, Z value of 2.87 and p-value < 0.00001. In the forest plot, an odds ratio value greater than 1 designates that the condition or event is more likely to occur. Since we also obtained an odds ratio value greater than 1, i.e., 2.25, it indicates that PRP is more effective for the treatment of knee osteoarthritis as compared to hyaluronic acid.
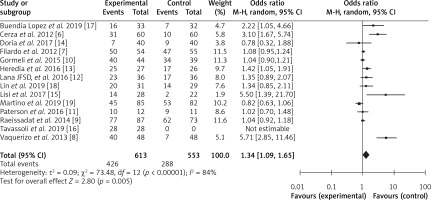
Relative risk was also calculated using RevMan software, and a forest plot was constructed, as shown in Figure 5. The pooled risk ratio was 1.34, with 95% CI ranging from 1.09 to 1.65. The risk ratio value of more than 1 suggests random sampling of data, use of categorical study variables with high performance, selection, and attrition bias. Since we also obtained a risk ratio value greater than 1, i.e., 1.34, it proves that PRP is safe and reduces the clinical symptoms and EQ-VAS score, IKDC subjective scores, KOOS, and WOMAC score of patients with fewer side effects. Heterogeneity was evaluated as a τ2 value of 0.09, χ2 value of 73.48, I2 value of 84%, Z value of 2.80 and p-value < 0.00001. The I2 value above 75% suggests that the PRP’s use in the treatment of knee osteoarthritis should be highly preferred. Similarly, a p-value less than 0.00001 means that all these results are highly statistically significant and favor the use of PRP for the treatment of knee osteoarthritis.
Combining all of these meta-analysis results, it is clear that PRP is a better alternative than hyaluronic acid and thus highly recommended for the treatment of knee osteoarthritis.
Discussion
Knee osteoarthritis (KOA) is a frequently reported musculoskeletal disorder among middle- and old-aged persons, characterized by deterioration and shortening of joint spaces and cartilage. Due to these deteriorations, patients have issues such as difficulty walking, limited motion, and less flexible movements. Diet control, rest, medication therapies, and knee arthroplasty are common treatment strategies. These strategies are aimed to either halt or stop the progression of knee osteoarthritis; however, no medication has been proven to do so completely. The existing treatment provides relief from pain, symptom remission, and function improvement. The commonly used medicines are oral glucosamine, chondroitin, acetaminophen, celecoxib, and chondroitin, but these drugs are usually associated with side effects, specifically liver issues. Arthroplasty can be a good substitute; still, it is not generally preferred due to its complicated surgical procedure.
Due to the shortcomings of the existing treatment procedure, various studies focus on applying PRP as an effective substitute and highly recommend its use. Since PRP is an autologous product made from a patient’s blood, it is entirely safe with no chance of graft rejection or any adverse inflammatory or allergic response. Furthermore, PRP consists of many growth factors and biologically active components; it promotes cell division fast and heals the deteriorated tissue.
For example, Cerza et al. in 2012 [6], Dai et al. in 2016, and di Martino et al. in 2019 [19], based on randomized controlled trials conducted by them, reported that in comparison to HA and saline, intra-articular injection of PRP is more beneficial for pain relief and functional improvement for osteoarthritis patients. Furthermore, in the meta-analysis conducted by Chen et al. in 2020 [20], and Karasavvidis et al. in 2020 [21], they also concluded that the use of PRP alone or in combination with hyaluronic acid is the safest and the best strategy for the treatment of knee osteoarthritis in patients of all age groups. Kon et al. (2020) also concluded that PRP may be superior to other supplements for pain reduction and functional recovery of knee osteoarthritis. However, in contrast to these results, some studies, e.g. Khosbin et al. in 2013 [22] and Han et al. in 2020 [23], reported no change in the patient’s clinical symptoms using the PRP treatment strategy and reported that lower efficacy limited use of PRP.
In the current meta-analysis, similar to the PRP supported studies, we also obtained the pooled odds ratio (OR) value of 2.55 with a 95% CI range of 1.35–4.84 with the τ2 value of 1.01, χ2 value of 52.79, I2 value of 77%, the Z value of 2.87 and p-value < 0.00001. The high odds ratio proved that PRP is a safe and effective strategy to reduce clinical symptoms with minimal side effects. The pooled risk ratio obtained was 1.34 to 95% CI ranging from 1.09 to 1.65 with a τ2 value of 0.09, χ2 value of 73.48, I2 value of 84%, the Z value of 2.80 and p-value < 0.00001. These values reflect the possibility of random sampling and high performance, selection, and attrition bias. However, a high I2 value above 75% highly supports the use of PRP for the treatment of knee osteoarthritis. The p-value of less than 0.00001 indicates statistically significant results and favors the use of PRP for the treatment of knee osteoarthritis.
Therefore, after a thorough systematic review and statistically significant meta-analysis results of the included randomized controlled trials (RCTs), the present meta-analysis highly favors PRP for the treatment of knee osteoarthritis.
The limitation of the present study is that the variability of control drugs used for the treatment of knee osteoarthritis in comparison to PRP skews the results. Similarly, considering different scores such as KOOS and WOMAC, and assessing clinical symptoms by different analytical tests performed by different persons have also influenced the risk of false-negative results. Furthermore, many studies have not reported the comparative efficiency of PRP with conventionally used HA, affecting the data to some extent. Data from other relevant studies showing the efficacy of PRP compared to hyaluronic acid can also include more results to suggest its use more precisely. Taking into account the variability, detailed data on the patient’s case history, physical examination, and pathological tests can provide further grounds for a recommendation of platelet-rich plasma as an effective treatment option for knee osteoarthritis.
In conclusion, although hyaluronic acid is widely used to treat knee osteoarthritis with a significant ability to lower the clinical symptoms, KOOS and WOMAC scores of patients, still, due to its strong and adverse side effects, it is not recommended. Instead, use of PRP (platelet-rich plasma) is preferred, proving to be an efficient and safe treatment strategy for patients with minimal side effects. Therefore, based on the current meta-analysis and statistically significant results (p < 0.00001), the use of PRP for the treatment of knee osteoarthritis in adults is highly recommended.