Type 2 diabetes mellitus (T2DM) is associated with increased prevalence of cardiovascular disease (CVD) [1–5]. Indeed, T2DM patients have a 2-4-fold higher risk for CVD morbidity and mortality than healthy non-diabetics [6]. Furthermore, CVD is the main cause of mortality in T2DM patients, accounting for almost 80% of deaths [7, 8]. The association between T2DM and CVD is supported not only by observational data and meta-analyses [9, 10], but also has a pathophysiological background based on the CV continuum that characterizes T2DM [11, 12]. The latter involves a chronic state of vascular inflammation, endothelial and platelet dysfunction, induced by hyperglycemia and insulin resistance that predisposes to macrovascular complications (i.e. CVD) even before T2DM diagnosis [13]. It has been reported that T2DM patients generally have coronary plaques with larger necrotic cores and greater inflammation (with more T lymphocytes and macrophages) as well as an increased rate of positive remodeling and plaque ruptures compared with nondiabetics controls, thus suggesting a more active atherosclerotic process [14, 15].
T2DM and CVD share a common pathogenesis (i.e. oxidative stress, inflammation and atherothrombosis), as well as common risk factors, including obesity, hyperinsulinemia, hypertension, dyslipidemia and non-alcoholic fatty liver disease [16–24]. Indeed, insulin resistance per se represents a major cause of CVD [25]. In this context, even “pre-diabetes” has been linked to increased risk of CHD events, stroke and all-cause death [26, 27]. Of note, there is a link between pre-diabetes, metabolic syndrome (MetS) and CVD risk [28, 29]. Similarly, MetS presence has been associated with both micro- and macrovascular complications in T2DM patients [30–33].
Historically, in 1998, Haffner et al. [34] evaluated the 7-year incidence of fatal or nonfatal myocardial infarction (MI) and stroke in a Finnish population cohort (n = 1,059 T2DM patients and 1,373 healthy non-diabetics). The 7-year rates of MI in T2DM patients with and without prior MI were 45.0 and 20.2%, respectively (p < 0.001); the corresponding rates for nondiabetics were 18.8 and 3.5%, respectively (p < 0.001) [34]. Furthermore, the 7-year incidence of stroke in T2DM patients with and without prior MI was 19.5 and 10.3%, respectively (p < 0.001); for nondiabetics the corresponding values were 7.2 and 1.9%, respectively (p = 0.01) [34]. Finally, 7-year CVD death rates were 42.0 and 15.4% for T2DM patients with and without prior MI (p < 0.001), whereas for nondiabetics they were 15.9 and 2.1%, respectively (p < 0.001). The 7-year CVD mortality rates remained similar between T2DM patients without prior MI and nondiabetics with prior MI, even after adjustment for age, gender, smoking, hypertension, triglyceride, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels [34]. These findings supported the notion that T2DM patients without known CHD have a similar CVD morbidity and mortality risk to nondiabetic CHD patients, thus highlighting the need for aggressive treatment of CVD risk factors in both these patient populations.
There has been a debate on whether T2DM (without known CVD) is a CHD equivalent or not, i.e. whether it is meaningful to consider the term “primary CVD prevention” in the presence of T2DM. In this context, it has been suggested that disease duration is important and that only T2DM patients with long disease duration, i.e. ≥ 10 years (without known CVD), could be regarded as CHD equivalent patients, thus having a similar risk of future CHD events compared with CHD patients without T2DM [6, 35]. In the 7-year cohort study by Haffner et al. [34] supporting the recognition of T2DM as a CHD equivalent, the mean T2DM duration was 8 years. Furthermore, when this Finnish population cohort was followed up for 18 years, CHD mortality remained similar between T2DM patients without MI and nondiabetics with prior MI, even after adjustment for several risk factors (i.e. age, gender, smoking, hypertension, total cholesterol, HDL-C, triglycerides) as well as T2DM duration [36]. Nevertheless, there are several studies showing that the earlier the onset and the longer the disease, the higher the CVD risk for T2DM patients [37, 38]. These findings are reasonable since atherosclerosis is an age-related disease and CVD risk increases with aging. However, the “real” clinical issue is not whether T2DM is or is not a CHD equivalent, but that T2DM patients (even without known CVD) are at a very high CVD risk, especially in the presence of CVD risk factors or target organ damage. This is clinically important since it can influence therapeutic strategies in T2DM patients without “diagnosed” CVD as well as adherence to treatment and clinical inertia [39]. In other words, why wait until a CVD event occurs in these very high-risk patients instead of preventing it?
The real diagnostic and therapeutic problem, however, is associated with the fact that, based on the European Society of Cardiology (ESC) risk categories, T2DM patients without target organ damage or other CVD risk factors are still classified as being at high CVD risk [40]. From the clinical point of view the question is whether we really see such patients in everyday practice. T2DM is usually diagnosed late, and in a large percentage of these patients, subclinical organ damage and/or other concomitant risk factors (e.g. dyslipidemia, overweight/obesity, hypertension) are already present [3]. Thus, while carefully investigating atherosclerosis risk factors and/or parameters of subclinical organ damage (similar to those recommended in patients with hypertension), there are very few T2DM patients who meet the criteria of those only at high CVD risk. So why simply not consider all T2DM patients as at very high CVD risk in order to start optimal, intensive, hypoglycemic, hypolipidemic and hypotensive treatment? We have enough data that such therapy may prevent CVD, being also cost-effective (see text below for details). The therapeutic approach of some diabetologists may still be too glucocentric, thus forgetting that T2DM patients are very likely to suffer a CVD event or death. Also, T2DM patients may be treated by general practitioners (GPs) worldwide, with only a small percentage of these patients being under the care of a diabetologist or a cardiologist. Therefore, it is important that all physicians who treat T2DM patients recognize their very high CVD risk.
Regarding recommendations from scientific societies, more than 2 decades ago, in 1993, the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) recognized T2DM as a CHD risk factor together with other established CVD risk factors including age, family history of premature CHD, smoking and hypertension [41]. In 2001, the NCEP ATP III stated that “diabetes is regarded as a coronary heart disease risk equivalent” [42]. Currently, as highlighted in a recent special Report from the American Heart Association (AHA)/American College of Cardiology (ACC), history of T2DM is included as a CVD predictor in different risk assessment tools such as the Framingham risk calculator, the ACC risk estimator and the AHA risk calculator [43]. The same Special Report recommends that individuals (without known CVD) with a coronary artery calcium (CAC) score of 0 should be treated with a statin only in the presence of T2DM, heavy cigarette smoking or family history of premature CVD, thus highlighting the clinical significance of T2DM in relation to CVD risk [43]. According to the 2019 ESC/European Association for the Study of Diabetes (EASD) guidelines, CAC scoring may be considered as a CV risk modifier in T2DM patients [44].
The latest (2019) American Diabetes Association (ADA) guidelines consider T2DM (without known CVD) in the presence of other CHD risk factors (including hypertension, smoking, albuminuria, chronic kidney disease (CKD), LDL-C ≥ 100 mg/dl (2.6 mmol/l) or family history of premature CVD) as a CHD equivalent [45]. Similarly, in the 2016 ESC/European Atherosclerosis Society (EAS) guidelines, T2DM patients (without a history of a CVD event) with target organ damage (e.g. proteinuria) or a major CVD risk factor (e.g. smoking, dyslipidemia or hypertension) are recognized as being at very high risk, i.e. in the same risk category as patients with known CVD [40]. Of note, left ventricular hypertrophy (LVH) represents another target organ damage that has been linked to CVD and renal dysfunction in T2DM patients [46–48]. However, the latest (2019) ESC/EAS guidelines categorize T2DM patients (without known CVD) with target organ damage (defined as microalbuminuria, retinopathy or neuropathy) or at least three major risk factors as at very high risk [49]. Similarly, in the 2019 ESC/EASD guidelines T2DM patients (without CVD history) with target organ damage (defined as proteinuria, eGFR ≥ 30 ml/min/1.73 m2, LVH or retinopathy) or at least three major risk factors (i.e. hypertension, dyslipidemia, smoking, obesity, age) are categorized as at very high risk [44]. Table I summarizes the recommendations from scientific societies regarding T2DM and CVD risk.
Table I
Summary of recommendations from scientific societies regarding type 2 diabetes mellitus and cardiovascular risk
| Scientific societies (year) [reference] | Recommendations |
|---|---|
| NCEP ATP III (2001) [42] | DM is a CHD risk equivalent |
| AHA/ACC Special Report (2019) [43] | Type 2 DM is included as a CVD predictor in different risk assessment tools such as the Framingham risk calculator, the ACC risk estimator and the AHA risk calculator |
| In the presence of type 2 DM, heavy cigarette smoking or family history of premature CVD, patients with CAC score 0 should be treated with a statin | |
| ADA (2019) [44] | DM patients with multiple coronary risk factors have a risk equivalent to that of patients with ASCVD |
| ESC/EAS (2016) [40] | Type 2 DM patients (without a history of CVD event) with target organ damage (e.g. proteinuria) or a major CVD risk factor (e.g. smoking, dyslipidemia or hypertension) are at very high risk, i.e. in the same risk category as patients with known CVD |
| ESC/EAS (2019) [49] | Type 2 DM patients (without a history of a CVD event) with target organ damage (microalbuminuria, retinopathy, neuropathy) or at least three major risk factors are at very high risk, i.e. in the same risk category as patients with known CVD |
| ESC/EASD (2019) [44] | Type 2 DM patients (without a history of a CVD event) with target organ damage (proteinuria, eGFR ≥ 30 ml/min/1.73 m2, left ventricular hypertrophy, retinopathy) or at least three major risk factors (hypertension, dyslipidemia, smoking, obesity, age) are at very high risk, i.e. in the same risk category as patients with known CVD |
[i] NCEP ATP III – Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), AHA – American Heart Association, ACC – American College of Cardiology, ADA – American Diabetes Association, EAS – European Atherosclerosis Society, ESC – European Society of Cardiology, DM – diabetes mellitus, CVD – cardiovascular disease, CHD – coronary heart disease, CAC – coronary artery calcium, ASCVD – atherosclerotic cardiovascular disease, eGFR – estimated glomerular filtration rate.
The ADA guidelines do not recommend screening for CHD in asymptomatic T2DM patients, partly because these patients are already at high risk and thus should be treated with intensive medical therapy [45]. These recommendations consider further investigations for CHD in the presence of electrocardiogram (ECG) abnormalities (e.g. Q waves), atypical cardiac symptoms (e.g. chest discomfort or unexplained dyspnea), carotid bruits, stroke, transient ischemic attack, claudication or peripheral artery disease (PAD). The critical question is how many patients with diagnosed T2DM indeed undergo this diagnostic process worldwide. A previous (2013) joint Task Force of the ESC/ADA recommends the performance of ECG, echocardiography, exercise test and Holter monitoring in T2DM patients without known CVD, based on clinical judgment [13]. In the 2019 ESC/EASD guidelines, a resting ECG is recommended in T2DM patients with hypertension or suspected CVD [45]. Of note, up to 60% of MIs in T2DM patients may be asymptomatic, thus being diagnosed only by ECG screening [13]. The prevalence of silent myocardial ischemia (SMI) is 20–35% in T2DM patients with additional CVD risk factors, and up to 70% of patients with SMI may also have significant coronary stenoses [13]. Furthermore, T2DM is related to an increased risk for sudden cardiac death [50, 51] and atrial fibrillation [52, 53]. With regard to peripheral arteries, the 2019 ESC/EASD guidelines consider the assessment of carotid (and/or femoral) plaque burden by ultrasonography as a risk modifier in asymptomatic T2DM patients [44]. In contrast, surprisingly, carotid intima-media thickness is not recommended by these guidelines for CV risk evaluation in T2DM patients [44].
Therefore, there is an urgent need to better define CHD screening in T2DM patients. A comprehensive foot evaluation should be performed at least annually in all T2DM patients, including measurement of the ankle-brachial index (ABI), especially in patients with decreased or absent pedal pulses or symptoms of claudication [45]. Of note, apart from a diagnostic tool for PAD, the ABI is considered a useful marker adding predictive value to the usual CVD risk estimation as supported by the ESC Working Group on peripheral circulation [54]. Since PAD is mostly asymptomatic (and thus frequently remains undiagnosed and untreated) and T2DM patients are more prone to PAD than the general population [55], ABI measurement should be performed in T2DM patients, as recommended by the joint 2013 Task Force of the ESC/ADA [13] and the 2019 ESC/EASD guidelines [44]. It should be noted that T2DM patients might frequently have high ABI values (i.e. > 1.3), mainly due to medial artery calcification, a characteristic feature of T2DM [56]. Such elevated ABI values also correlate with CVD morbidity and death [56], as also highlighted by the 2019 ESC/EASD guidelines [44]. According to current AHA/ACC [57] and ESC/EASD guidelines [44], the toe-brachial index (or Duplex ultrasound [44]) should be measured in such patients with non-compressible arteries to diagnose PAD.
Multifactorial treatment is critical in T2DM patients (with or without known CVD). The Steno-2 study in 2008 showed that multifactorial intervention (targeting glucose, blood pressure, lipids and platelets) significantly reduced CVD events and death as well as total mortality in 160 T2DM patients with microalbuminuria [58]. Another recent cohort study (n = 144,271 T2DM patients without history of CVD) found that the greatest reduction in CVD events was achieved when hemoglobin A1c, blood pressure and LDL-C were all optimally controlled [59]. The 2019 ESC/EASD guidelines mention that the combined reduction of HbA1c, systolic blood pressure and lipids can decrease CVD events by 75%, and that multifactorial treatment remains underused [44].
Regarding T2DM patients without known CVD, statins can prevent CVD events [60], as shown in the Collaborative Atorvastatin Diabetes Study (CARDS) [61], the Heart Protection Study (HPS) [62] and the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Lipid Lowering Arm (LLA) [63]. This is also supported by available meta-analyses [64, 65]. In terms of antihypertensive drugs, a fixed combination of perindopril and indapamide was reported to significantly lower the rate of micro- and macrovascular events in T2DM patients, irrespectively of the presence or absence of CVD at baseline in the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) trial [66]. Ramipril also significantly decreased CVD morbidity and mortality as well as all-cause death in T2DM patients with or without known CVD in the Heart Outcomes Prevention Evaluation (HOPE) study [67]. Similar results were obtained with losartan in T2DM patients with LVH (but without known CVD) in the Losartan Intervention For Endpoint reduction (LIFE) study [68]. Of note, fixed combinations of antihypertensive drugs and/or polypills containing aspirin, statin and one or more antihypertensive medication may represent a cost-effective strategy for CVD prevention, also improving patients’ adherence to treatment and thus achievement of therapeutic goals [69–71].
With regard to antidiabetic drugs, in the recently published Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial [72], dulaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA) administered subcutaneously once weekly, was shown to significantly reduce the composite endpoint of non-fatal MI, non-fatal stroke or CVD death compared with placebo in both groups of T2DM patients (i.e. with known CVD (31.5%) or with CVD risk factors); this benefit was mainly attributed to a decrease in non-fatal stroke occurrence. Semaglutide, another subcutaneously administered GLP-1 RA once weekly, significantly lowered the rates of the primary outcome (MI, stroke or CVD mortality) compared with placebo in T2DM patients with or without known CVD in the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) [73]; the observed benefit was largely attributed to stroke prevention. In subgroup analyses, the semaglutide-related reduction of the composite endpoint was similar in T2DM patients with known CVD or with CVD risk factors only (17% of the total study population [73].
Among sodium-glucose co-transporter-2 inhibitors (SGLT2i), canagliflozin was reported to significantly decrease the composite of CVD morbidity and mortality, as well as hospitalization for heart failure (HF), compared with placebo in T2DM patients in the Canagliflozin Cardiovascular Assessment Study (CANVAS) program [74]. Of note, subgroup analyses showed a similar CVD benefit between T2DM patients with (65.6%) or without a history of CVD at baseline [74]. However, canagliflozin use was related to a doubled risk of amputation in the lower extremities compared with placebo. This side effect was observed only with canagliflozin and not with other SGLT2i (e.g. empagliflozin or dapagliflozin), and thus it should be taken into consideration by physicians who treat T2DM patients [45, 75]. In the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE–TIMI 58) trial [76], dapagliflozin significantly lowered the rate of HF hospitalization compared with placebo in T2DM patients with (40.6%) or without known CVD, as shown in subgroup analyses.
The BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) [77] was the first positive CVD outcome trial with a SGLT2i, thus introducing a new era in the treatment of T2DM patients. In this trial, empagliflozin use was associated with a significant reduction in the composite of CVD morbidity and mortality, as well as HF hospitalizations, but also significantly decreased CVD and all-cause death compared with placebo in T2DM patients with known CVD at baseline [77]. Interestingly, all empagliflozin-related CVD benefits (i.e. composite of CVD morbidity and death, as well as HF hospitalization, total and CVD mortality) were consistent in patients with or without a prior atherothrombotic event at baseline (i.e. stroke or MI) [78], thus highlighting its clinical usefulness in T2DM patients without known MI or stroke. Of note, a report from the ESC Cardiovascular Roundtable in 2018 [79], as well as the 2019 ESC/EASD guidelines, recommends the use of SGLT2i or GLP-1 RAs with proven CVD benefit as monotherapy in drug-naïve T2DM patients with known CVD or at high/very high risk [44].
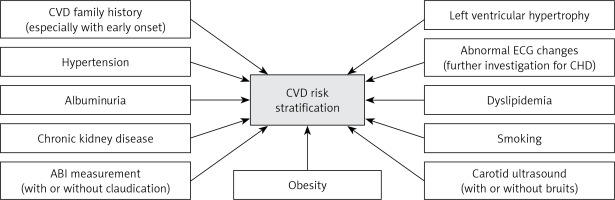
Based on the above, T2DM patients should be individually evaluated, in terms of CVD risk stratification, regarding CVD family history (especially with early onset), hypertension, dyslipidemia, smoking, obesity, CKD, albuminuria, LVH, claudication, abnormal ABI, carotid bruits (and carotid ultrasound) and abnormal ECG changes (Figure 1). It is however a great challenge to select those risk factors which should be relatively easy to measure and evaluate also for the non-specialist, such as GPs or internal medicine physicians, especially in the light of knowledge of limited diabetologists’ accessibility for T2DM patients. In the presence of such CVD risk factors, T2DM should be treated as a CHD equivalent, irrespective of disease duration. It follows that in such patients all CVD risk factors should be aggressively treated. Furthermore, the selection of antidiabetic drugs should focus on preventing CVD morbidity and mortality.
Figure 1
Evaluation of patients with type 2 diabetes mellitus in terms of cardiovascular risk stratification
CVD – cardiovascular disease, CHD – coronary heart disease, ABI – ankle-brachial index, ECG – electrocardiogram.
Conflict of interest
NK has given talks, attended conferences and participated in trials sponsored by Amgen, Astra Zeneca, Boehringer Ingelheim, Elpen, Mylan, NovoNordisk, PharmaSwiss, Sanofi, Servier and WinMedica. DPM has given talks and attended conferences sponsored by Amgen, AstraZeneca and Libytec. MB has received research grants/support from Sanofi and Valeant, and has served as a consultant for Abbott/Mylan, Akcea, Amgen, KRKA, NovoNordisk, MSD, Polfarmex, Polpharma, Sanofi-Aventis, Servier, Esperion, and Resverlogix.


