Introduction
Laminaria japonica, the most important economic seaweed for edible-medicinal use, has high nutritional value and health functions [1]. Traditionally, it is widely consumed as a marine vegetable for humans in many eastern Asian countries [2]. Moreover, it has been used in traditional Chinese medicine (TCM) as a home remedy for phlegm elimination, detumescence and weight loss for over one thousand years [3]. Over the past decades, L. japonica has drawn the attention of chemists and pharmacologists on account of the abundance of functional compounds and their biological properties [4]. Many studies have recently suggested that polysaccharides were the main active components in L. japonica [5]. Polysaccharides, one of the main classes of bioactive substances from fungi, algae, and higher plants, have been demonstrated to exhibit a wide range of pharmacological activities, including broad immunomodulatory and antitumor effects [6, 7]. Polysaccharide intake stimulates the immune system and improves survival in cancer patients [8]. Laminarins, which are polysaccharides in L. japonica extracts, have been reported to have immunomodulatory activities, which can enhance the phagocytic and secretory activity of macrophages and induce the production of reactive oxygen species (ROS), nitric oxide (NO), and cytokines (TNF-α, IL-1, and IL-6) [9].
NK cells were initially identified due to their ability to kill tumor cell lines in vitro [10]. They exert a rapid and non-specific response upon stimulation by tumor cells and virus-infected cells as part of the body’s first line of defense, the innate immune system [11]. They are also implicated in adaptive responses to antibody-marked cells via antibody-dependent cellular cytotoxicity, as well as possessing the ability to stimulate T cells into effector T cells via release of interferon-γ [12]. However, the immunomodulatory effect of laminarin on NK cells is not yet fully reported.
The present study treated immunosuppressed mice with laminarin and then observed the activity of NK cells in the blood, and the levels of IL-12 and IFN-γ in serum prior to and following laminarin treatment. In addition, the cytotoxicity and the expressions of perforin and granzyme in the NK-92 MI cells were detected in vitro. The aim of this study was to investigate the molecular mechanism underlying NK cell activation by laminarin, and to provide a basis for the study of the immunoregulatory activity of laminarin.
Material and methods
Preparation of laminarin solution
Laminarin (purity > 96%) was purchased from Fortune BIO-tech Co., Ltd (Shanghai, China) and was dissolved in PBS.
Animal maintenance
Male balb/c mice (18-22 g, 4 weeks old) were obtained from Liaoning Changsheng Biotechnology Co., Ltd (Liaoning, China). During the experimental period, the mice were housed in a room maintained under a 12 h light/dark cycle at 24°C. Mice had free access to standard laboratory pellet chow and fresh water.
Animal treatment
The mice were randomly assigned to four groups with 10 mice per group, A: normal control group, B: cyclophosphamide (cy) model group, C: cy plus low-dose laminarin group, D: cy plus high-dose laminarin group. Mice of groups B, C and D were injected intraperitoneally with 50 mg of Cy (Shanxi Pude pharma, China)/kg on days 1-3. From the 4th day, the C and D groups were given laminarin followed by 500 and 1000 mg/kg by gavage for 10 days, and the A and B groups were given PBS by gavage. On the 14th day, peripheral blood cells were obtained by heart puncture, and then the mice were sacrificed by cervical dislocation and the spleens were collected for analysis. All experimental procedures were conducted according to the guidelines provided by the ethical committee of experimental animal care at Liaoning University of Traditional Chinese Medicine (Shenyang, China).
NK cell preparation
Cells from spleen were pooled and single-cell suspensions were prepared. Purified splenic natural killer cell populations were further isolated using MACS magnetic bead separation technology. Briefly, Anti-NK cell DX5 MicroBeads were used according to the manufacturer’s instructions (Miltenyi Biotec 130-052-501, Bergisch Gladbach, Germany) using the positive selection program PosselD on the autoMACS Pro Separator (Miltenyi, Bergisch Gladbach, Germany). Purity of cells were routinely tested by FACS and ranged from 87 to 91%.
ELISA analysis
The serum levels of IFN-γ and IL-12 were quantified using the double antibody sandwich ELISA kit (Uscn Life Science Inc., China). The assay was performed according to the manufacturer’s instructions. PBS was used as a blank control. Cytokine concentrations in the respective samples were determined on the basis of standard curves, prepared using recombinant cytokines of known concentrations.
Cell culture
NK92-MI cells were obtained from American Type Culture Collection (ATCC) and passaged several times in our laboratory. Cells were cultured in alpha modification of Eagle’s minimum essential medium (a-MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM L-glutamine, 0.2 mM inositol, 0.02 mM folic acid, 0.01 mM 2-mercaptoethanol, 12.5% FBS and 12.5% horse serum (Sigma-Aldrich Corporation, St. Louis, MO, USA).
Quantification of NK cell cytotoxicity
The isolated NK cells or NK92-MI cells (1 × 105 cells) were placed in each well of a 96-well plate in 50 µl of RPMI-1640 medium (Gibco), and then co-cultured with K562 (target) cells at an effectors-to-targets (E : T) ratio of 4:1 for 4 h.
The plates were centrifuged, and 100 µl of supernatants were transferred to fresh 96-well plates. Subsequently, an LDH-substrate mixture (100 µl, content of the kit, Roche) containing the tetrazolium salt INT was added to the supernatants. After incubation for 30 min in dark at room temperature, absorbance was determined at a wavelength of 492 nm against a reference wavelength of 600 nm. Lysis was calculated using the following equation:
Western blot analysis
The cells were washed three times with PBS and then lysed in RIPA buffer in the presence of proteinase inhibitor cocktail (Roche). The protein concentration was determined using a BCA assay (Bios, Beijing, China). Aliquots (25 mg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with primary antibodies against perforin and granzyme (Abcam, Cambridge, UK) at 4°C for 12h, washed extensively with 0.1% Tween-20 in PBS, and incubated with secondary antibodies conjugated to horseradish peroxidase (Proteintech Group, Inc. Wuhan, China) at 1:10000 dilutions. The membranes were washed and visualized by an enhanced chemiluminescence (ECL) detection system (Bio-Rad Laboratories). Bands from experiments were quantified by densitometry using Image J software (image.nih.gov/ij/), and results were normalized to β actin expression in each sample.
Flow cytometry and analysis
Flow cytometry was used for analysis of surface activating receptors stained with CD134 (NKG2D)-FITC, CD337 (NKp30)-PE, CD336 (NKp44)-PerCP, CD335 (NKp46)-APC (eBioscience, MN, USA).
The cells were washed, re-suspended to 5 × 105 cells/ml in ice-cold PBS supplemented with 0.5% BSA, incubated with 10 µl of conjugated antibodies for 40 min at 4°C, washed twice in PBS/0.5% BSA and analyzed directly using a FACScan flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA). An unstained cell suspension was prepared in PBS, which acted as a negative control for FACS. We counted 10,000 events per sample, and the percentages of cells with natural cytotoxicity receptors (NCRs) were obtained by single-color analysis. Data were analyzed using FlowJo software (TreeStar Inc.).
Statistical analysis
Results are expressed as the mean± standard deviation (SD). The difference among groups was analyzed with SPSS statistical software (20.0; IBM, Corps., Armonk, NY, USA) using oneway analysis of variance with the posthoc Fisher’s least significant difference test. All experiments were performed in triplicate. Statistical significance was considered to be p<0.05.
Results
Effects of laminarin on NK cell cytotoxicity of immunosuppressive mice
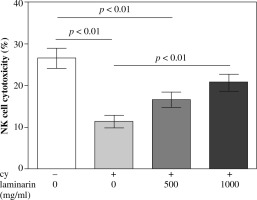
Effects of laminarin on NK cell cytotoxicity of immunosuppressive mice are shown in Figure 1. NK cell cytotoxicity of cy model group mice is significantly lower than that of the normal control group (p < 0.01). Compared to the cy model group, the NK cell cytotoxicity of the cy plus high-dose laminarin group was significantly higher (p < 0.01). Compared to the normal control group, NK cell cytotoxicity of the cy plus low-dose laminarin group was significantly lower (p < 0.01), but there was no significant difference between the cy plus high-dose laminarin group and the normal control group (p > 0.05).
Effects of laminarin on IL-12 and IFN-a level in serum of immunosuppressive mice
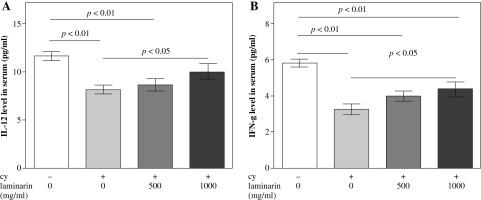
Effects of laminarin on IL-12 and IFN-γ of immunosuppressive mice are shown in Figure 2. IL-12 and IFN-γ levels in serum of cy model group mice are both significantly lower than that of the normal control group (p < 0.01). Compared to the cy model group, IL-12 and IFN-γ levels in serum of the cy plus high-dose laminarin group were significantly higher (p < 0.05). Compared to the normal control group, IL-12 level in serum of the cy plus low-dose laminarin group was significantly lower (p < 0.01), but there was no significant difference between the cy plus high-dose laminarin group and the normal control group (p > 0.05). Compared to the normal control group, the IFN-γ level in serum of both cy plus low-dose and high-dose laminarin groups was significantly lower (p < 0.01).
Effects of laminarin on NK92-MI cell cytotoxicity
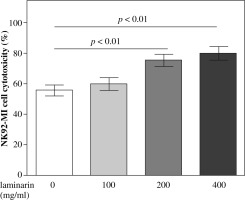
Effects of laminarin on NK92-MI cells cytotoxicity are shown in Figure 3. Compared to the control group, 200 mg/ml and 400 mg/ml laminarin both increased the NK92-MI cell cytotoxicity significantly (p < 0.01).
Effects of laminarin on perforin and granzyme B expression of NK92-MI cells
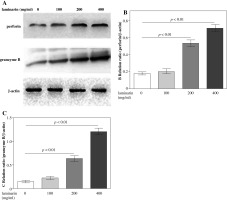
Effects of laminarin on perforin and granzyme B expression of NK92-MI cells are shown in Figure 4. Compared to the control group, 200 µg/ml and 400 µg/ml laminarin both increased perforin and granzyme B expression of NK92-MI cells significantly (p < 0.01).
Effects on expression of activating receptors on NK92-MI cells of laminarin
Effects on expression of activating receptors on NK92-MI cells of laminarin are shown in Figure 5. Compared to the control group, 100 µg/ml, 200 µg/ml and 400 µg/ml laminarin all increased NKp30 and NKG2D expression on NK92-MI cells significantly (p < 0.01).
Discussion
Natural killer cells are major effector cells of the innate immune system which are located in the first line of defense and resisting tumor and virus infection of our body [13]. While killing target cells, NK cells do not have to be sensitized by antigens in advance and have no MHC restriction [14]. NK cells are capable of lysis and apoptosis of target cells by releasing perforin and granzyme [15, 16]. They regulate anti-tumor and anti-viral activity by releasing cytokines such as IFN-γ [17].
In patients with cancer, radiotherapy and chemotherapy cannot avoid injury to the immune system, which mainly manifests in the following two aspects: radioactivity has a directly cytotoxic action on immune effector cells, and the drugs of chemotherapy can directly or indirectly kill immunologic effector cells, both leading to decreased immune function [18, 19]. Given that NK cells play critical roles in the first line of defense against malignancies by direct and indirect mechanisms [20], the therapeutic use of NK cells in human cancer immunotherapy has been proposed and followed in a clinical context [21].
Laminaria japonica is known for its richness in polysaccharides, minerals and certain vitamins. The most abundant polysaccharides in Laminaria japonica are laminarin, fucoidan and alginic acid [22, 23]. Laminarin can boost the immune system, reduce cholesterol levels in serum, and lower systolic blood pressure [24, 25]. Studies have shown that laminarin can induce activation of RAW 264.7 cells, the murine macrophage cell line, and bone-marrow-derived DCs (BMDCs) [26]. Laminarin can induce DC maturation and Ag specific Th1 and CTL activation that can effectively kill Ag-expressing B16 melanoma cells in vivo [27]. The immune stimulatory function of laminarin will be potentially useful for developing immunotherapy reagents for human use.
In the test, compared to the normal control group, the cytotoxicity of NK cells and IL-12, IFN-γ level in serum in the immunosuppressive model group were all reduced significantly, showing that the immunosuppressive model of mice was made successfully. Compared to the cy model group, high-dose laminarin increased the cytotoxicity of NK cells and IL-12, IFN-γ level in serum significantly, showing that laminarin could improve NK cell cytotoxicity of immunosuppressive mice. IL-12 and IFN-γ play a pivotal role in regulating cell-mediated immunity, which are essential for NK cell activation [28]. IFN-γ production by NK cells has been identified as an integral part of NK-cell cytotoxic activity [29]. Activated NK cells are the main source of IFN-γ [30]. IL-12 is a key inducer of NK-cell IFN-γ secretion and cytotoxicity [31].
In vivo laminarin increase of NK cell cytotoxicity may be related to two factors: on the one hand, laminarin can increase IL-12 and IFN-γ levels in serum, and on the other hand, laminarin may increase NK cell cytotoxicity directly. In the present study, we investigated the regulation of NK cell functions by laminarin using human NK cell line NK92-MI cells in vitro.
Compared to the normal control group, 200 µg/ml and 400 µg/ml laminarin increased the cytotoxicity and expression of perforin and granzyme significantly. This showed that the mechanism of promotion of NK cell cytotoxicity of laminarin may be related to the ability of laminarin to promote the expression and release of perforin and granzyme.
Natural cytotoxicity receptors are receptors that mediate NK cell activation and found only on NK cells. Their surface density is correlated with the magnitude of NK cytotoxicity against target cells [32]. Cytotoxicity against most target cells requires the participation of a combination of activating receptors [33]. In this report, we provide experimental evidence that the expression and function of activating receptors in NK-mediated killing are regulated by laminarin. We showed that laminarin upregulated the expression of NKp30 and NKG2D. These results suggest that laminarin-stimulated cytotoxicity of NK92-MI cells against K562 cells may depend on upregulation of NKp30 and NKG2D.