Introduction
Laparoscopy-assisted D2 total gastrectomy for upper gastric cancer has undergone extensive development and gained popularity because of the advantages of minimally invasive surgery, including lower intraoperative blood loss, less postoperative pain, faster recovery, better cosmetic outcomes, fewer complications and more acceptable oncological outcomes than open gastrectomy [1–4]. However, locoregional recurrence often occurs in patients with local advanced gastric cancer (AGC), despite an R0 resection. Several reports have suggested that the dissemination of cancer cells is the main reason for tumor relapse [5, 6]. Moreover, Xie et al. [7] demonstrated the existence of disseminated cancer cells in the mesogastrium, termed “metastasis V”, and concluded that this phenomenon may be a risk factor for locoregional recurrence. Since en bloc resection of the primary lesion and its adjacent tissues is the gold standard of radical surgery, conventional D2 total gastrectomy, during which lymphadenectomy is performed based on the presence of blood vessels in adipose or connective tissues, seems to be unsatisfactory in preventing residual tumor or cancer cell dissemination.
According to previous studies, total mesorectal excision or complete mesocolic excision has been widely used for colon and rectal cancer, and the oncological prognosis is significantly improved [8–10]. However, there is another less common procedure to treat gastric cancer. Although some surgeons have attempted D2 + complete mesogastrium excision (CME) or total gastrectomy through the outside bursa, the standard surgical plane and technique for CME are still unclear [11, 12]. In contrast to the free part of the stomach, the posterior part of the stomach is composed of complex anatomical structures, including multiple fascias of the perigastric mesogastrium, intricate vascular networks and peripheral organs. Thus, perigastric mesogastrium separation remains a technically challenging aspect of CME, and an appropriate approach is necessary. Our previous study has demonstrated the “enjoyable space” (the latent intrafascial space between the left Gerota’s fascia and the posterior aspect of the lesser peritoneal sac), and it might be a novel, minimally invasive space and approach, respectively, to achieve CME and provide benefit for the dissection of lymph nodes (LNs) no. 10 and no. 11 [13]. Therefore, we proposed the “enjoyable space” approach to separate the whole perigastric mesogastrium following latent intrafascial spaces and achieve D2 + CME radical en bloc resection.
Aim
The aim of this study is to describe the “enjoyable space” approach and to investigate its safety and feasibility relative to conventional D2 total gastrectomy.
Material and methods
Embryological background and surgical plane for perigastric mesogastrium excision
Early in embryonic development, the stomach is covered by mesenteries, consisting of double layers of peritoneum, suspended in the middle. The peritoneum between the stomach and the posterior abdomen wall is called the dorsal mesogastrium (DM), in which the pancreas, spleen, and celiac trunk and its branches are encapsulated. With the development of the embryo, the stomach undergoes a 90-degree rotation around the craniocaudal axis, and the DM folds downwards and forms two layers (anterior and posterior), each with two leaves. The large sac between the anterior layers and the posterior layers is known as the omental bursa. Next, the anterior leaf of the posterior layers of the DM, which encompasses the pancreas, evolves into the anterior pancreatic fascia (APF), whereas the posterior leaf of the posterior layers of the DM is fused with the primitive transverse mesocolon to form the anterior lobe of the transverse mesocolon (ALTM) and with the posterior abdominal wall to form the posterior peritoneum. The posterior peritoneum covering the left kidney is called the left Gerota’s fascia. During this evolution, the mesentery fuses closely with the mesentery, blood vessels, peripheral organs and posterior abdominal wall to form fusion fascia [14, 15] (Figure 1). In addition, the section of mesenteries between the stomach and peripheral organs forms ligaments: to the transverse colon as the gastrocolic ligament (GCL), to the spleen as the gastrosplenic ligament (GSL) and to the celiac trunk and pancreas as the gastropancreatic fold (GPF). The fusion fascia is widely distributed in the perigastric mesogastrium. The intrafascial space within the fusion fascia is a latent avascular zone filled with loose connective tissue. Thus, this space can be used as a potential anatomical plane for perigastric mesogastrium excision.
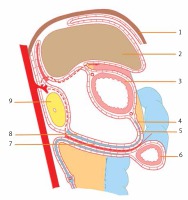
Figure 1
Schematic representation of the layers of the perigastric mesogastrium and the operative path. 1 – diaphragm, 2 – liver, 3 – stomach, 4 – anterior leaf of the anterior layers of the greater omentum, 5 – posterior leaf of the anterior layers of the greater omentum, 6 – transverse colon, 7 – posterior leaf of the posterior layers of the greater omentum, 8 – anterior leaf of the posterior layers of the greater omentum, 9 – pancreas. The dashed red line shows the operative path
In this study, we attempted to identify the surgical plane along the intrafascial space. The entire surgical plane was preliminarily divided into two regions: the superior region and the inferior region. Due to the presence of the GPF, the superior region was divided into two compartments: the superior recess in the right, and the splenic recess in the left [16]. For better understanding, we divided the surgical plane into three parts: the inferior region, the superior recess and the splenic recess. The inferior region includes the ALTM and the surface of the pancreas, extending right to the lateral border of the duodenum and left to the inferior pole of the spleen. In the superior region, the surgical plane followed the surface of the common hepatic artery (CHA) and its branches, the posterior peritoneum in the superior recess, and the left Gerota’s fascia in the splenic recess (Figure 2).
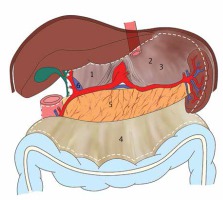
Figure 2
Schematic representation of the surgical plane and surgical scope for perigastric mesogastrium excision. 1 – superior recess, 2 – splenic recess, 3 – left Gerota’s fascia, 4 – anterior lobe of the transverse mesocolon, 5 – surface of the pancreas, 6 – portal vein. The dashed white line shows the surgical scope for perigastric mesogastrium excision
Patients
A total of 165 patients with advanced upper gastric cancer underwent laparoscopy-assisted radical total gastrectomy between January 2015 and December 2017 and were included in this study. All patients were subjected to preoperative examination, including endoscopic examination and endoscopic biopsy specimen analysis, computed tomography (CT) scanning, and abdominal ultrasonography (US). The patients were divided into two groups based on the surgical approaches: the conventional D2 total gastrectomy group (D2 group, n = 81) and the D2 + CME total gastrectomy group (D2 + CME group, n = 84). The clinicopathological characteristics, surgical outcomes and postoperative complications were postoperatively collected and analyzed according to the surgical video and complete medical records. To improve accuracy, we measured intraoperative blood loss by estimating the volume of blood in the suction container and weighing blood-soaked gauze, confirmed the tumor location, and measured the maximum diameter immediately after surgery. LN grouping was performed according to the Japanese gastric cancer treatment guidelines (ver. 4) [17]. The depth of tumor (T), nodal status (N) and tumor stage (TNM) were determined according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual [18]. The benefits and risks of the procedures were explained in detail to both patients and their families, and written informed consent was obtained. This study (approval number: 20080323) was approved by the Ethics Committee of the Affiliated Hospital of Putian University.
The inclusion criteria were as follows: preoperative examinations confirming upper gastric cancer; tumor depth of T2-T4a; no preoperative evidence of enlargement or involvement of LNs or distant metastasis; no simultaneous operation in other organs; surgery performed by the same surgeon; and curative resection (R0) according to the postoperative pathological diagnosis.
The exclusion criteria were as follows: prior abdominal surgery or preoperative chemoradiation therapy; tumor depth of T1 or T4b; intraoperative evidence of peritoneal dissemination or distant metastasis; observation of enlargement or involvement of perigastric LNs during operation; simultaneous operation in other organs; and incomplete pathological data. Patients with preoperative or intraoperative enlargement or involvement of LNs underwent open surgery and were not included in this study.
Surgical technique
Laparoscopic conventional D2 total gastrectomy
The conventional D2 approach for laparoscopic total gastrectomy was similar to that reported in the literature [19]. The procedure can be briefly described as follows: The GCL was divided to free the great omentum. In the infrapyloric area, the right gastroepiploic vein (RGEV) and the right gastroepiploic artery (RGEA) (LN no. 6) were divided at their origin. Next, the GPF was exposed, and the left gastric vein (LGV) and the left gastric artery (LGA) (LN no. 7) were vascularized and divided. Subsequently, LNs along the celiac trunk (LN no. 9) and the CHA (LN no. 8a) were removed. In the suprapyloric area, the right gastric artery (RGA) (LN no. 5) was vascularized and divided. Along the border of the liver, the lesser omentum was dissected, and LNs in the anterior region of the hepatoduodenal ligament (HDL) (LN no. 12a) were removed. At the superior border of the pancreas, the fatty lymphatic tissues along splenic vessels (LN no. 11) and around the splenic hilum (LN no. 10) were completely removed. The left gastroepiploic artery, posterior gastric artery (PGA), and all short gastric vessels were divided, and the corresponding LNs were removed (LN no. 4sa and 4sb). Before gastric transaction, the right cardiac LNs (LN no. 1) and left cardiac LNs (LN no. 2) were dissected en bloc. After the laparoscopic operation, an omega-type Roux-en-Y digestive tract reconstruction was performed using circular staplers.
The “enjoyable space” approach for D2 + CME total gastrectomy
Step 1. Separating the inferior region: The GCL was routinely divided to locate the ALTM (Photo 1 A). The surgical plane was extended along the ALTM toward the right to expose the lateral border of the duodenum and toward the left to expose the tail of the pancreas and the inferior pole of the spleen (Photos 1 B and E). Then, the gastrocolic fusional fascia was separated to expose and divide the RGEV and the RGEA (LN no. 6) at their origin (Photos 1 C and D). Subsequently, the APF was gently peeled toward the superior border of the pancreas to expose the gastroduodenal artery (GDA) and the origin of the LGA and splenic artery (SA) (Photo 1 F).
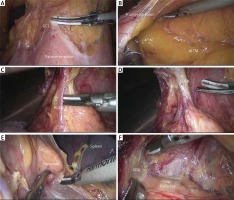
Photo 1
An intraoperative image showing the procedure of separating the inferior region. A – Dividing the gastrocolic ligament. B – Locating and extending along the anterior lobe of the transverse mesocolon. C – Exposing the right gastroepiploic vein. D – Exposing the right gastroepiploic artery. E – Exposing the tail of the pancreas and the inferior pole of the spleen. F – Peeling the anterior pancreatic fascia toward the superior border of the pancreas
GCL – gastrocolic ligament, ALTM – anterior lobe of the transverse mesocolon, RGEV – right gastroepiploic vein, RGEA – right gastroepiploic artery, GDA – gastroduodenal artery.
Step 2. Separating the superior recess: The perigastric fascia along the GDA and CHA was separated to create room for exposing and dividing the RGA, and the anterior region of the HDL was peeled along the proper hepatic artery (PHA) (Photos 2 A–D). Then, suprapyloric LNs (LN no. 5) and LN no. 12a were removed. Separation was continued from the CHA upwards to expose the portal vein (PV) (Photo 2 E). Thus, the main vascular networks within the superior recess were exposed and vascularized to maintain the integrity of the perigastric fascia. After separating the splenic recess, the residual perigastric fascia of the superior recess could be dissected from the GPF toward the right to complete the separation of the superior recess (Photo 2 F).
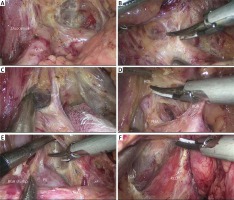
Photo 2
An intraoperative image showing the procedure of separating the superior recess. A – Separating the perigastric fascia along the gastroduodenal artery. B – Separating the perigastric fascia along the common hepatic artery. C – Exposing the right gastric artery. D – Peeling the hepatoduodenal ligament from the proper hepatic artery. E – Exposing the portal vein. F – Dissecting the residual perigastric fascia of the superior recess from the gastropancreatic fold toward the right
GDA – gastroduodenal artery, CHA – common hepatic artery, RGA – right gastric artery, PHA – proper hepatic artery, HDL – hepatoduodenal ligament, PV – portal vein, CV – coronary vein, LGA – left gastric artery, RCD – right crus of the diaphragm.
Step 3. Separating the splenic recess: The intrafascial space of the splenic recess was referred to as the “enjoyable space” in our previous study [13]. The procedure can be briefly described as follows: The entrance: Exposing the origin of the LGA and SA, the loose connective tissue within the entrance, defined by the LGA on the right side and the SA on the lower side, was cut to obtain access to the splenic recess and to locate the left Gerota’s fascia (Photo 3 A). Next, the dissection was meticulously continued along the surface of the left Gerota’s fascia to expand the surgical plane. The lower border: exposing the profile of the SA, the membrane-like tissue along the surface of the SA was dissected toward the tail of the pancreas (Photo 3 B). The left border: pushing the posterior gastric wall upward to reveal the incisal margin, the surgical plane was continuously extended along the surface of the left Gerota’s fascia toward the left to fully expose the posterior edge of the middle-upper spleen (Photo 3 C). The right border: in the GPF, the right crus of the diaphragm (RCD) was denuded from the back of the LGA to the right side of the esophageal hiatus (EH) (Photo 3 D). The upper border: pushing the posterior wall of the body and fundus of the stomach upward to reveal the upper perigastric space, the posterior gastric mesentery was dissected from the surface of the crura of the diaphragm to expose the EH and the gastrophrenic ligament (GPL) (Photo 3 E). Last, the celiac trunk (LN no. 9) and the LGA (LN no. 7) were completely skeletonized, and the LGA was divided at its root (Photo 3 F). Thus, the separation of the splenic recess was completed.
Photo 3
An intraoperative image showing the procedure of separating the splenic recess. A – Exposing the entrance. B – Locating the left Gerota’s fascia and exposing the splenic vessels. C – Exposing the posterior edge of the middle-upper spleen along the left Gerota’s fascia. D – Denuding the right crus of the diaphragm. E – Exposing the esophageal hiatus. F – Skeletonizing the celiac trunk and the left gastric artery
LGA – left gastric artery, CHA – common hepatic artery, SA – splenic artery, SV – splenic vein, EH – esophageal hiatus, RCD – right crus of the diaphragm, LCD – left crus of the diaphragm, PHA – proper hepatic artery, GDA – gastroduodenal artery.
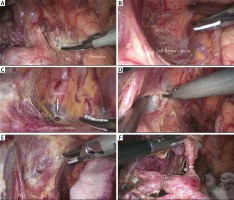
Step 4. Dissection of the surrounding mesenteries and ligaments: The surrounding mesenteries and the GSL were successively pulled up to expose the splenic vessels and its branches. The fatty lymphatic tissue around the splenic vessels was dissected along the surface toward the splenic hilum (Photo 4 A). Meanwhile, the PGA and left gastroepiploic vessels (LGEVs) (LN no. 4sb) were divided from their origin (Photo 4 B). Subsequently, the inferior splenic lobar vessels (ISLVs) and superior splenic lobar vessels (SSLVs) were gradually skeletonized to complete splenic hilar lymphadenectomy (Photos 4 C and D). In this procedure, 3–4 branches of the short gastric arteries (SGAs), which originate from SSLVs, were divided at their roots. Then, the GPL could be easily exposed and cut away (Photo 4 E). At the lesser curvature, the HGL was dissected along the lower border of the liver, and the LNs around the lesser curvature (no. 3) were removed (Photo 4 F). Finally, the phrenoesophageal membrane and both vagus nerves were divided to fully expose the lower esophagus and dissect LNs no. 1 and no. 2. At this point, the “enjoyable space” approach was completed.
Photo 4
An intraoperative image showing the procedure of dissection of the surrounding mesenteries and ligaments. A – Skeletonizing the splenic artery and dividing the posterior gastric artery. B – Exposing the left gastroepiploic vessels. C – Skeletonizing the inferior splenic lobar vessels. D – Skeletonizing the superior splenic lobar vessels. E – Dissecting the gastrophrenic ligament. F – Dissecting the hepatogastric ligament
SA – splenic artery, PGA – posterior gastric artery, LGEVs – left gastroepiploic vessels, ISLVs – inferior splenic lobar vessels, SSLVs – superior splenic lobar vessels, GPL – gastrophrenic ligament, HGL – hepatogastric ligament.
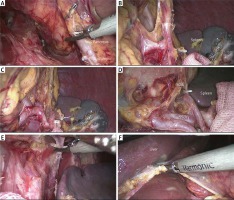
After the laparoscopic operation, gastric transaction and omega-type Roux-en-Y digestive tract reconstruction were performed similarly to conventional D2 total gastrectomy.
Statistical analysis
All statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). Data were reported as the means ± standard deviations (SDs). Unpaired Student’s t tests, χ2 test or Fisher’s exact test was used to compare continuous variables and categorical variables. A p-value of less than 0.05 was considered statistically significant.
Results
Clinicopathological characteristics of included patients
The clinicopathological characteristics of the 165 patients are listed in Table I. The cohort consisted of 137 (83.0%) males and 28 (17.0%) females with a mean age of 63.1 ±8.9 years (range: 29–80 years). The mean body mass index (BMI) was 22.54 ±3.24 kg/m2 (range: 14.36–34.72 kg/m2), and the tumor size was 5.1 ±1.5 cm (range: 2.8–11.0 cm). Sex, age, body mass index (BMI), tumor location, tumor size, tumor depth (pT), lymph node metastasis (pN) and TNM stage did not differ between the groups (p > 0.05 each).
Table I
Comparisons of clinicopathological characteristics between the groups
Surgical outcomes of the D2 group vs. the D2 + CME group
All patients successfully underwent laparoscopic-assisted radical total gastrectomy (with conventional D2 or D2 + CME total gastrectomy). No patient required conversion to laparotomy and splenectomy, and no operation-related death occurred during the perioperative period. For all 165 patients, the mean operative time was 230.8 ±28.4 min (range: 160–300 min), the mean blood loss was 81.6 ±43.4 ml (range: 30–260 ml), and the median number of retrieved LNs was 32.0 ±8.2 (range: 19–61) per patient. The operative time was significantly prolonged, and the mean blood loss was significantly reduced in the D2 + CME group (p < 0.05 each). The mean number of retrieved LNs was slightly higher in the D2 + CME group than in the D2 group (p < 0.05). In contrast, the mean time to first flatus, liquid diet, and soft diet and the duration of hospital stay were similar between groups (p > 0.05 each) (Table II).
Table II
Comparisons of surgical outcomes between the groups
Postoperative complications of the D2 group vs. the D2 + CME group
The overall postoperative morbidity rate among all patients was 12.7% (21/165). The rates of postoperative complications were not different between the D2 group and the D2 + CME group (16.0% (13/81) vs. 9.5% (8/84), p > 0.05) (Table III). We observed four cases of pulmonary infection, two cases of paroxysmal atrial fibrillation, one case of postoperative delirium, two cases of incision infection, one case of lymphatic leakage, one case of abdominal hemorrhage with exploratory laparotomy to achieve hemostasis, and one case of delayed SA pseudoaneurysm sixteen days after surgery successfully treated with an arterial intervention in the conventional D2 group. In contrast, we observed three cases of pulmonary infection, two cases of paroxysmal atrial fibrillation, one case of postoperative delirium, and two cases of incision infection in the D2 + CME group. All patients with postoperative complications were successfully treated and discharged. Both intraoperative and postoperative 30-day mortality rates for all patients were 0%.
Table III
Comparisons of postoperative complications between the groups
Follow-up
Of all the 165 patients, 160 (96.9%) patients were followed up from 12 to 48 months (median: 29 months), including 78 patients (96.3%, median: 38 months, range: 30–48 months) in the D2 group and 82 patients (97.6%, median: 20 months, range: 12–30 months) in the D2 + CME group. Forty-four patients died of advanced tumors, including 23 patients in the D2 group and 11 patients in the D2 + CME group, during the follow-up period.
Discussion
D2 lymphadenectomy has become globally accepted as the standard surgical procedure for curable AGC, with increasing studies of long-term oncological outcomes in eastern and western countries [20–22]. However, whether CME can contribute to LN dissection and benefit patients with AGC remains uncertain. Our study suggested that the “enjoyable space” approach may be an optional procedure to achieve CME, improve the quality of surgery and increase the number of harvested LNs.
The “enjoyable space” approach is a complicated, technique-dependent and time-consuming procedure that improves short-term outcomes. Our data showed that the mean blood loss in the D2 + CME group was significantly lower than that in the D2 group (71.4 ±41.2 ml vs. 92.2 ±43.3 ml, p < 0.05) and that the mean number of retrieved LNs was slightly higher in the D2 + CME group than in the D2 group (33.2 ±8.4 vs. 30.7 ±7.6, p < 0.05). Several factors may be associated with these results. First, because the intrafascial space is a latent avascular zone filled with loose connective tissue, it not only is convenient to expand during the operation but also rarely causes unexpected hemorrhage. Second, the entire procedure can be continuously performed along the surgical plane out of the omental bursa, and the perigastric mesogastrium can be dissected en bloc. In contrast to conventional D2 lymphadenectomy, in CME, the supporting vascular and lymphatic systems of the posterior wall of the stomach can be maximally excised. Moreover, the blood vessels can be comfortably exposed at different levels for dissecting LNs after separating the perigastric intrafascial space. These factors can contribute to increase the number of harvested LNs. Finally, in this study, all operations were performed by the same experienced surgeon, ensuring both operational stability and data comparability. However, due to the complicated anatomical structure of the perigastric mesogastrium, the separation of the superior recess and splenic recess is technically demanding, and surgical landmarks and procedural separation techniques are necessary for smooth operation. In the superior recess, the blood vessels play an important role as surgical landmarks. We first peeled the perigastric fascia from the surface of the GDA and CHA to create room to expose the PHA and RGA. Subsequently, the perigastric fascia was continuously separated upward to skeletonize the PHA and expose the PV. Then, the perigastric fascia of the superior recess could be easily separated from the posterior peritoneum. In this manner, it was beneficial not only to dissect LNs no. 8 and no. 12 but also to maintain the integrity of the perigastric mesogastrium. In the splenic recess, the left Gerota’s fascia is a good continuous surgical plane, and the origin of the LGA and SA can be identified as a reference mark of the starting point to obtain access to the splenic recess. The “enjoyable space” can be smoothly extended for complete hollowing of the perigastric space, which may provide more room and a better view for subsequent splenic hilar lymphadenectomy. Nevertheless, because of the obviously complicated procedure of the “enjoyable space” approach, the mean operative time of the D2 + CME group was correspondingly prolonged (237.4 ±27.1 min vs. 223.8 ±28.3 min).
The assessment of postoperative complications is necessary for evaluating a novel technique. In our study, the overall postoperative morbidity was 12.7%, which was consistent with that in the relevant literature [23–27]. Some similar studies on CME have suggested that bursectomy does not increase the risk of postoperative morbidity [23, 28]. Accordingly, our data showed that although the postoperative complication rate in the D2 + CME group was slightly lower than that in the D2 group (9.5% (8/84) vs. 16.0% (13/81)), there was no significant difference between the two groups, suggesting that the “enjoyable space” approach was safe and did not increase the postoperative complication rate.
There are several limitations in this study. First, it was a single center retrospective study that lacked randomized large-scale controlled clinical trials. Second, due to the insufficient follow-up period, the long-term oncological prognosis has not yet been assessed. Last, cases with prior abdominal surgery and preoperative chemoradiation therapy were not included in this study. Therefore, the range of application of this technique is not sufficient.
Conclusions
The “enjoyable space” approach has a longer operative time, lower intraoperative blood loss, and a slightly higher number of harvested LNs than the conventional method. Therefore, this approach might be safe and feasible to achieve D2 + CME. However, randomized large-scale controlled clinical trials are needed to validate the data and to evaluate the long-term oncological efficacy.









