Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
THYROID DISEASE / BASIC RESEARCH
MiR-376c-3p targets heparin-binding EGF-like growth factor (HBEGF) to inhibit proliferation and invasion in medullary thyroid carcinoma cells
1
Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
2
Department of Endocrinology, Affiliated Hospital of Jiangnan University, Jiangsu, China
3
Department of Endocrinology, Huai’an Second People’s Hospital, Huai’an, China
Submission date: 2018-09-10
Final revision date: 2018-12-18
Acceptance date: 2019-01-03
Online publication date: 2019-05-21
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):878-887
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Aggressive medullary thyroid carcinomas (MTC) have a high mortality rate and the treatment for patients diagnosed with advanced MTC is comparatively ineffective. We hence aimed to test the effects of miR-376c-3p on MTC and to explore the relevant mechanism.
Material and methods:
Cell Counting Kit-8 (CCK-8) and soft agar colony formation assay were applied to evaluate the proliferation of transfected MZ-CRC-1 cells. Wound healing and transwell assay were employed to evaluate MTC cell migration and invasion, respectively. Luciferase assay was performed to validate the downstream target of miR-376c-3p in MZ-CRC-1 cells. Quantitative polymerase chain reaction was used to detect mRNA abundance of key genes. Western blot technique was used to analyze protein levels of HBEGF, E-cadherin, ZO-1, N-cadherin and vimentin.
Results:
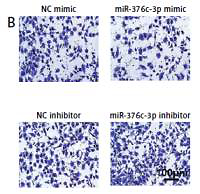
MiR-376c-3p inhibited the viability, migration and invasion of MZ-CRC-1 cells. Moreover, miR-376c-3p mimic downregulated expression of N-cadherin and vimentin but upregulated that of E-cadherin and ZO-1 in MZ-CRC-1 cells. Results for the luciferase reporter assay showed that miR-376c-3p was able to bind the 3 untranslated region of heparin-binding EGF-like growth factor (HBEGF), of which overexpression nearly nullified the miR-376c-3p mimic-induced inhibitory effects in the MTC cells.
Conclusions:
MiR-376c-3p showed suppressive effects on MZ-CRC-1 cells via targeting and downregulating HBEGF, suggesting that miR-376c-3p could potentially be targeted for the treatment of MTC.
Aggressive medullary thyroid carcinomas (MTC) have a high mortality rate and the treatment for patients diagnosed with advanced MTC is comparatively ineffective. We hence aimed to test the effects of miR-376c-3p on MTC and to explore the relevant mechanism.
Material and methods:
Cell Counting Kit-8 (CCK-8) and soft agar colony formation assay were applied to evaluate the proliferation of transfected MZ-CRC-1 cells. Wound healing and transwell assay were employed to evaluate MTC cell migration and invasion, respectively. Luciferase assay was performed to validate the downstream target of miR-376c-3p in MZ-CRC-1 cells. Quantitative polymerase chain reaction was used to detect mRNA abundance of key genes. Western blot technique was used to analyze protein levels of HBEGF, E-cadherin, ZO-1, N-cadherin and vimentin.
Results:
MiR-376c-3p inhibited the viability, migration and invasion of MZ-CRC-1 cells. Moreover, miR-376c-3p mimic downregulated expression of N-cadherin and vimentin but upregulated that of E-cadherin and ZO-1 in MZ-CRC-1 cells. Results for the luciferase reporter assay showed that miR-376c-3p was able to bind the 3 untranslated region of heparin-binding EGF-like growth factor (HBEGF), of which overexpression nearly nullified the miR-376c-3p mimic-induced inhibitory effects in the MTC cells.
Conclusions:
MiR-376c-3p showed suppressive effects on MZ-CRC-1 cells via targeting and downregulating HBEGF, suggesting that miR-376c-3p could potentially be targeted for the treatment of MTC.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.