Introduction
Ovarian cancer is one of the most common malignancies worldwide [1, 2]. Each year approximately 140,000 people in the world die from ovarian cancer [3]. To date, staging/debulking surgery and taxane-platinum-based chemotherapy are commonly used treatment modalities for ovarian cancer [4]. Despite advances in surgical techniques and chemotherapy, the 5-year survival rate for ovarian cancer is still less than 50% [5, 6]. This poor prognosis is due to latent symptoms in the early stages, tumor resistance to chemotherapy, and a lack of effective early detection [7]. In order to improve the poor survival of ovarian cancer, a large number of studies are needed to unravel the molecular mechanism(s) responsible for the development of ovarian cancer.
MicroRNAs (miRNAs) are non-coding RNAs, composed of 20–23 nucleotides. miRNA binds to the 3′-UTR of a target gene and plays a negative regulatory role by inhibiting mRNA translation or inducing mRNA degradation [8, 9].
Accumulating studies indicated that miRNAs were involved in the progression of ovarian cancer and therefore could be used for cancer detection [10]. miRNAs have been reported to inhibit tumorigenesis of ovarian cancer by suppressing cell proliferation [11], migration [12], invasion [13, 14], and epithelial-to-mesenchymal transition [15]. In addition, miRNAs were also expected to be an important diagnostic tool [16, 17] as well as therapeutic targets for ovarian cancer [18, 19]. Although a few studies have suggested that miR-331-3p can promote the growth of liver cancer [20, 21], miR-331-3p was taken as a suppressor in a variety of other human cancers. Studies showed that miR-331-3p could inhibit cervical cancer [22], gastric cancer [23], renal cell carcinoma [24], and colorectal cancer [25] by promoting apoptosis, suppressing cell proliferation, and inhibiting migration and invasion of tumor cells. However, the mechanism of miR-331-3p is still unclear in ovarian cancer.
The results of the current study showed that by inhibiting the target gene RCC2, miR-331-3p could significantly suppress tumor metastasis in an ovarian cancer cell line.
Material and methods
Cell lines
Of six human ovarian cancer cell lines, A2780 and SKOV3 were purchased from ATCC (Manassas, USA), and CAOV3, OVCAR3, ES-2, and COC1 were purchased from the Cell Center of Chinese Academy of Medical Sciences (Beijing, China). Two immortalized ovarian surface epithelial (IOSE) cells, IOSE80 and IOSE144, were purchased from BeNa Culture Collection (Suzhou, China). The 293T cell line was purchased from ATCC (Manassas, USA). Cells were cultured in appropriate growth media supplemented with 10% FBS and antibiotics at 37°C in presence of 5% CO2 following the manufacturer’s instructions.
Reverse transcription-PCR (RT-PCR)
The total RNA was extracted from cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The primers for miR-331-3p and U6 were provided by the miRNA Real-Time PCR Assay kit (Genepharm, China). The primers for GAPDH and RCC2 were obtained from Sangon Biotech (Shanghai, China): GAPDH, forward, 5′-GCACCGTCAAGGCTGAGAAC- 3′, reverse, 5′-ATGGTGGTGAAGACGCCAGT- 3′; RCC2 forward 5′-TTCCTTTGGGTGCCCTGAA- 3′, reverse 5′-GGCAGAATCTGTCCATCTTTCG- 3′. The expression of RCC2 was detected by qRT-PCR (SYBR Green PCR kit, Bio-Rad). The relative expression level was determined by the 2–ΔΔCt method. U6 and GAPDH were used as controls. Each experiment was performed 3 times.
Transfection of oligonucleotide
Negative control (NC) mimics, miR-331-3p mimics, and miR-inhibitors were designed and synthesized by Genepharm (Shanghai, China). Cells were transfected with NC mimics, miR-331-3p mimics or miR-inhibitors using transfection reagent (pufei biotech, China) following the manufacturer’s instructions.
RCC2 overexpressing stable cells
The RCC2 encoding sequence (without 3′-UTR) was cloned and inserted into the pcDNA3.1(+) vector (Invitrogen). Cell transduction was performed with Lipofectamine 2000 (Invitrogen) following the manufacturer’s protocol, and RCC2 overexpressing stable cells were selected in the presence of 500 μg/ml of G418. The pcDNA3.1(+) vector was transfected into SKOV3 and A2780 cells and stable cell lines were selected as controls as described above.
Cell proliferation
Cell proliferation was measured with the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). The absorbance at 450 nm was measured at 0 h, 24 h, 48 h, and 72 h; and then the growth curves were established to evaluate proliferation rates. Each experiment was performed 3 times.
Wound-healing assay
Cells were cultured in six-well plates and grown to 90% confluency. A 10 μl pipette tip was used to make a wound on cell monolayers and then the cells were cultured in medium supplemented with 10% FBS. Wound healing was observed using a microscope (Leica, German) at 0 h, 24 h, 48 h, and 72 h. The wound healing rate was calculated using image pro plus 6.0 software.
Migration and invasion assay
Cell migration and invasion were measured by transwell assay. Briefly, cells were trypsinized, washed and seeded on standard 24-well chambers (Corning Costar) at a seeding density of 1 × 105 cells per well, and FBS was added to the bottom chamber as a chemo-attractant. After 24 h, chambers were fixed and stained with crystal violet, and the migrated cells were counted using a microscope. The invaded cells were counted after 48 h. Data are shown as the mean value ± SD from triplicate experiments.
Luciferase assays
A psiCHECK-2 vector (Promega) which contained the luciferase reporter gene and the 3′-UTR of RCC2 (or its mutant sequence) was transfected into the 293T cell, and then miR-331-3p mimics or the control mimics were transfected into the same cells. After 48 h, the cells were cracked and the firefly and Renilla fluorescence was detected by the Dual-Luciferase Reporter Assay System (Promega). Renilla fluorescence reflected the expression of 3′-UTR of RCC2. The experiments were performed independently in triplicate.
Western blot
The total protein was extracted from cells using NP-40 lysis buffer, supplemented with protease inhibitor cocktail (Roche Applied Science). A BCA protein quantitative kit (Beyotime) was used to detect protein concentration. The primary antibodies anti-RCC2 (Abcam) and anti-GAPDH (Abcam) were used in the western blot assays. All the experiments were performed in triplicate independently.
Results
miR-331-3p is down-regulated in ovarian cancer
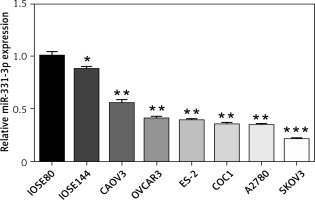
To examine the expression of miR-331-3p in human ovarian cancer cells, total RNAs were isolated from six ovarian cancer cell lines (CAOV3, OVCAR3, ES-2, COC1, A2780 and SKOV3), and two immortalized ovarian surface epithelial cell lines (IOSE80 and IOSE144). Quantitative-PCR was performed to measure miR-331-3p expression. The expression levels of miR-331-3p were down-regulated in ovarian cancer cell lines, compared to that of the normal ovarian surface epithelial cells. Decreased miR-331-3p expression in ovarian cancer cell lines suggested miR-331-3p to be a tumor suppressor in ovarian cancer (Figure 1).
Figure 1
Expression of miR-331-3p in ovarian carcinoma. The relative expression levels of miR-331-3p in six EOC cell lines (CAOV3, OVCAR3, ES-2, COC1, A2780, and SKOV3) and two immortalized ovarian surface epithelial cells (IOSE80, IOSE144) were determined by qRT-PCR. *p < 0.05, **p < 0.01, ***p < 0.001
miR-331-3p inhibits cell proliferation, migration and invasion
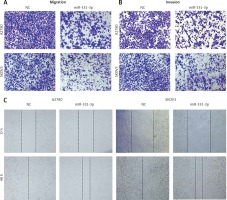
In order to confirm the impact of miR-331-3p in ovarian cancer, miR-331-3p mimics were transfected into ovarian cancer cell lines A2780 and SKOV3. The expression of miR-331-3p was detected by RT-PCR. The results showed that miR-331-3p was upregulated in transfected cells (Figure 2 A). Proliferation assays showed that miR-331-3p expression significantly inhibited cell proliferation in A2780 and SKOV3 cells (Figure 2 B). Transwell assays were utilized to examine the impact of miR-331-3p on EOC cell migration and invasion. The results showed that miR-331-3p mimics dramatically inhibited the migration abilities of SKOV3 and A2780 cells (Figure 2 C). Similarly, the invasion abilities of SKOV3 and A2780 cells were suppressed significantly after transfection with miR-331-3p mimics compared to that of cells transfected with NC mimics (Figure 2 D). Cell mobility in A2780 and SKOV3 was determined by wound-healing assays (Figure 2 E). The results showed that miR-331-3p mimics suppressed the cell mobility significantly in A2780 and SKOV3 cells. Images of transwell and wound healing assays are shown in Figure 3. These data suggested that miR-331-3p suppresses cell proliferation, migration and invasion of ovarian cancer.
Figure 2
miR-331-3p suppresses EOC cell proliferation, migration and invasion. A – miR-331-3p level was detected by qRT-PCR. B – Proliferation of EOC cells treated with miR-331-3p or NC mimics was measured using Cell Counting Kit-8 (CCK-8). C – Transwell migration assay of EOC cells treated with miR-331-3p or NC mimics. D – Transwell invasion assay of EOC cells treated with miR-331-3p or NC mimics. E – Wound healing assays of EOC cells transfected with miR-331-3p or NC mimics
miR-331-3p directly targets RCC2
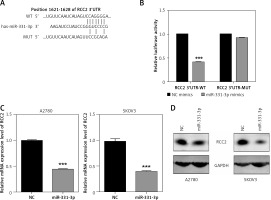
In order to characterize the molecular mechanism by which miR-331-3p suppresses EOC growth, migration and invasion, we searched three target scan databases – TargetScan, DIANA and miRanda – which predicted RCC2 as a direct target of miR-331-3p (Figure 4 A). To confirm that miR-331-3p can target and suppress RCC2, a luciferase reporter vector containing the binding site of RCC2 3′-UTR, wild type (WT) or the mutant type (MUT) was constructed and transfected into 293T cells. The results of luciferase assays are shown in Figure 4 B . In the WT group, miR-331-3p significantly suppressed the relative luciferase activities, whereas in the mutant type group, no significant difference in the relative luciferase activity was detected between NC and miR-331-3p treated cells. The results of the luciferase assay suggested that miR-331-3p suppresses RCC2 by identifying and binding specific sequences of RCC2 3′-UTR. The mRNA level (Figure 4 C) and protein level (Figure 4 D) of RCC2 in miR-331-3p transfected A2780 and SKOV3 were detected by RT-PCR and western blot respectively. The results showed that the expression of RCC2 was significantly inhibited by miR-331-3p at both the mRNA level and the protein level.
Figure 4
RCC2 is a direct target of miR-331-3p in EOC cells. A – Predicted binding sites of miR-331-3p in the 3′-UTR of RCC2. MUT (Mutant-type) was generated by mutating four nucleotides in the seed region of the 3′-UTR of RCC2. B – Luciferase report assays were performed in 293T cells, which were transfected with RCC2 3′-UTR WT or MUT vectors, and treated with miR-331-3p or NC mimics. C – The mRNA expression of RCC2 in A2780 and SKOV3 cells after 48 h transfection with miR-331-3p and NC mimics was measured by qRT-PCR. D – The protein level of RCC2 in A2780 and SKOV3 cells after 48 h transfection with miR331-3p and NC mimics was measured by western blot
Restoration of RCC2 expression rescues the impact of miR-331-3p on EOC cells
To explore whether RCC2 is the target of miR-331-3p in ovarian cancer; RCC2 was overexpressed in A2780 and SKOV3 cells by transfection with recombinant vectors carrying RCC2 cDNA (without 3′-UTR) (Figure 5 A). Transwell and wound healing assays were conducted, and the results showed that the subsequent reintroduction of RCC2 without an intact 3′-UTR partly restored the inhibitory effects of miR-331-3p mimics on the cell proliferation, migration and invasion ability of A2780 and SKOV3 cells (Figures 5 B–E, 6). Taken together, the data indicate that RCC2 acts as a functional target of miR-331-3p in EOC cells.
Figure 5
Overexpression of RCC2 reverses the effect of miR-331-3p on EOC cells. A – Overexpression of RCC2 in A2780 and SKOV3 was confirmed by qRT-PCR. B – Proliferation of A2780 and SKOV3 was measured using Cell Counting Kit-8 (CCK-8) assays. C – Transwell migration assay of EOC cells treated with miR-331-3p or NC mimics. D – Transwell invasion assay of EOC cells treated with miR-331-3p or NC mimics. E – Wound healing assays of EOC cells treated with miR-331-3p or NC mimics
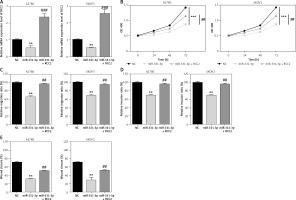
Figure 6
Overexpression of RCC2 restores the migration and invasion of EOC cells. Images of Figure 5 C (A), Figure 5 D (B), and Figure 5 E (C) are shown

Discussion
Ovarian cancer is one of the world’s most common gynecological malignant tumors [5, 26]. Despite recent advances in detection and cytotoxic therapies, more than 70% of ovarian cancer cases were diagnosed at an advanced stage, and late diagnosis led to poor prognosis [27, 28]. Furthermore, patients with ovarian cancer are faced with a serious risk of recurrence, and the current standard treatment is unable to cure the disease [6, 29]. Therefore, ovarian cancer still remains the leading cause of mortality in gynecologic cancer, which accounts for nearly 140 thousand deaths per year worldwide [5, 26]. Thus, there is an urgent demand to develop new diagnostic and therapeutic targets for this devastating disease. In recent years, more and more miRNAs have been found to play an important role in the occurrence and development of ovarian cancer.
miR-331-3p was reported to suppress a wide variety of human cancers by inhibiting different target genes. In human renal carcinoma cells, miR-331-3p inhibits cell proliferation by targeting HER2 and NRP2 [24]; miR-331-3p has also been reported to inhibit cell proliferation, block the cell cycle and induce apoptosis in cervical cancer by regulating NRP2 [22], while in prostate cancer, miR-331-3p suppresses cell proliferation and migration by directly targeting RALA [30].
Our study found that miR-331-3p was significantly reduced in ovarian cancer cells compared to immortalized ovarian surface epithelial cell lines (Figure 1). These results suggested that miR-331-3p might act as a suppressor in ovarian cancer. In order to verify this hypothesis, miR-331-3p mimics were transfected into EOC cells (Figure 2 A). CCK8, transwell, and wound healing assays were conducted, and the results indicated that miR-331-3p suppressed EOC cell proliferation, migration, and invasion (Figures 2 B–E). In addition, regulator of chromosome condensation 2 (RCC2) was predicted to be a novel target of miR-331-3p. The luciferase assay verified that miR-331-3p negatively regulated RCC2 gene expression via specifically binding to the 3′-UTR (Figures 4 A, B). Results of RT-PCR and western blot suggested that the expression of RCC2 was downregulated by miR-331-3p in EOC cells (Figures 4 C, D). In addition, upregulated RCC2 was able to restore the cell proliferation, migration, and invasion in miR-331-3p transfected cells (Figure 5). The results in Figure 5 suggested that RCC2 plays an important role in the miR-331-3p suppressing ovarian cancer. RCC2 was previously reported to be an important component of the chromosomal passenger complex (CPC); and CPC was regarded as an important regulator of chromosome and cytoskeleton formation as well as mitosis [31, 32]. In recent years, RCC2 has been reported as a tumor promoter in various cancers. RCC2 was shown to be upregulated in gastric carcinoma tissues, which can regulate the cell cycle process to promote the proliferation of cancer cells [33, 34]. Another study reported that RCC2 knockdown arrested the cell cycle at prometaphase in HeLa cells, suggesting RCC2 to be a negative regulator of the cell cycle which was found to be driven by regulating chromosomal separation and cell division [35–37]. The molecular mechanism of RCC2 in ovarian cancer will be further verified in our future work.
In conclusion, our study revealed that miR-331-3p could significantly inhibit the proliferation, migration and invasion of EOC cells, and that RCC2 is the direct target of miR-331-3p in ovarian cancer. The findings from our study further clarify the role of miR-331-3p in ovarian carcinoma tumorigenesis, and demonstrate that the miR-331-3p /RCC2 signaling axis could be a novel tumor detection marker as well as a potential therapeutic target for ovarian carcinoma.