Introduction
The use of microRNAs (miRs) for cancer therapy is currently one of the promising fields in cancer research [1]. The miRs are around 18–25 nucleotide long endogenous molecules that control the expression of the target genes by binding to 3´UTR causing degradation of mRNA or repression of translation [2]. The miRs play vital roles in fundamental cellular processes which include but are not limited to proliferation, development, differentiation, apoptosis and autophagy [3]. There is strong evidence that many miRs are aberrantly expressed in cancerous tissues and play an important role in the development of cancer [4]. Hence, it is believed that miRs may serve as therapeutic targets and will allow targeted therapy for the treatment of cancer [5]. MiR-575 has been shown to regulate the proliferation of different types of cancers such as non-small lung carcinoma and gastric cancer [6, 7]. Nonetheless, there is no study that reports the role of miR-575 in thyroid cancer. Thyroid cancer is one of the most prevalent types of endocrine cancers [8]. It accounts for 1 to 1.5% of all newly diagnosed cancers in the United States [8]. Although the incidence of most neck and head cancers has decreased over the last few decades, the incidence of thyroid cancer is still steeply increasing. Several factors which include both genetic and environmental have been held responsible for the increasing incidence of thyroid cancer [9]. Moreover, recent studies have indicated thyroid cancer to be more common in women than in men [10]. Surgical interventions, chemotherapy and radiotherapy are employed for the treatment of thyroid cancer [11]. Nonetheless, the late diagnosis due to the dearth of biomarkers and lack of therapeutic targets obstructs the treatment of thyroid cancer [12].
Herein we for the first time report that miR-575 is downregulated in thyroid cancer tissues and cell lines and regulates the proliferation, migration and invasion of thyroid cancer cells by targeting Derlin 1. Taken together, these results suggest that miR-575 acts as a tumor suppressor in thyroid cancer and may point to a novel therapeutic target in thyroid cancer.
Material and methods
Cell transfection
Thyroid cancer tissues and cell lines
The thyroid cancer tissues and the adjacent normal tissues were obtained from the Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, from June 2017 to June 2018. The thyroid cancer cell lines (MDA-T32, TT, K1 and MDA-T68) and the normal thyrocytes were obtained from the Cancer Research Institute of Beijing (Beijing, China) and maintained in Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies, Massachusetts, USA) supplemented with 10% fetal bovine serum (Invitrogen Life Technologies, Massachusetts, USA), 100 μg/ml streptomycin and 100 U/ml penicillin G (Himedia, Pennsylvania, USA) in an incubator at 37°C with 5% CO2.
cDNA synthesis and qRT-PCR
The TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA) was used for the extraction of RNA from the tissues and cell lines. Subsequently, the total RNA was reverse-transcribed by a RevertAid cDNA synthesis kit (Fermentas). Thereafter quantitative RT-PCR was performed using SYBR Green master mix (Applied Biosystems; Foster City, Calif., USA) on an ABI 7900HT system. All reactions were carried out in triplicate and the gene expression was determined by the 2–ΔΔCt method.
Transfections
The miR-575 mimics and miR-NC were ordered from RiboBio (Guangzhou, China). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was then used to perform the transfections in accordance with the manufacturer’s guidelines. As the MDA-T32 and MDA-T68 cells reached 80%, the appropriate concentrations of miR-575 mimics or miR-NC were transfected into these cells.
MTT assay
The MDA-T32 and MDA-T68 cells were seeded in a 96-well tissue-culture plates with approximately 2500 cells/well. The viability of the cells was evaluated at different time intervals by Vybrant MTT Cell Proliferation Assay (Invitrogen, USA) as the manufacturer’s guidelines. Finally, the optical density was measured at 570 nm.
Analysis of cell death
The transfected MDA-T32 and MDA-T68 cells were cultured for 24 h at 37°C and then fixed with ethanol (70%) for 20 min. The cells were then subjected to PBS washing and subsequently stained with DAPI. Finally, the cells were examined under a microscope to detect the induction of apoptosis. The MDA-T32 and MDA-T68 cells were transfected with appropriate constructs and then incubated for 48 h at 37°C. The cells were then dissociated with the help of trypsin and then washed with phosphate buffered saline (PBS). The cells were then resuspended in 1X binding buffer which was followed by the addition of 5 µl of annexin V-FITC and propidium iodide (PI). The cell culture was then placed in a dark room for 15 min. The apoptosis percentage was then evaluated by a flow cytometer.
Wound healing assay
The transfected MDA-T32 and MDA-T68 cells were placed in twelve-well plates with approximately 1 × 105 cells per well. A scratch was made by a pipette tip at 24 h after transfection. The cells were washed with PBS and fresh medium was added. Following 24 h incubation at 37°C, the cells were subjected to fixation with methanol. The initial wound width and final wound width were determined from photomicrographs.
Cell invasion assay
Transwell chambers with Matrigel were employed to monitor invasion of the MDA-T32 and MDA-T68. In brief, the cells were transfected with appropriate constructs and 48 h after transfection the cells were harvested and suspended in fresh medium. While 200 µl of the cell suspension containing approximately 5 × 104 cells was placed into the upper compartment, 500 µl of fresh medium was placed in the lower compartment. After 24 h cells present in the upper compartment were removed by swabbing while cells that invaded the lower surface were fixed and then subsequently stained with 0.05% crystal violet. Finally, ten random fields were selected to determine the invasion under the light microscope.
Western blotting
The thyroid cancer tissues and cell lines were lysed and the protein concentration in each sample was measured by Bradford assay. Equal concentrations of the proteins from each sample were loaded on 10% SDS polyacrylamide gels (SDS-PAGE), followed by shifting to polyvinylidene fluoride membranes. Blocking of the membrane was then performed by fat-free milk (5%) in TBST. This was followed by incubation with a primary antibody for 24 h at 4°C. Subsequently, a secondary antibody was added at 25°C for about 2 h. The bands of interest were finally observed by chemiluminescence.
Statistical analysis
The experiments were carried out in three biological replicates. The data are presented as the mean the replicates ± SD. The statistical analysis was carried out by Student’s t test or one way ANOVA followed by Tukey’s test. P < 0.05 was considered to indicate statistical significance.
Results
MiR-575 is downregulated in thyroid cancer
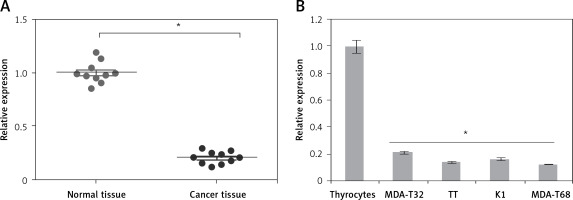
The gene expression of miR-575 was examined in thyroid cancer tissues and normal adjacent tissues of 10 patients. The qRT-PCR results showed that expression of miR-575 was significantly suppressed in thyroid cancer tissues (Figure 1 A). Gene expression analysis of miR-575 in thyroid cancer cells showed miR-575 to be significantly (up to 8.5) downregulated in thyroid cancer cell lines (Figure 1 B). The highest downregulation was observed in MDA-T68 and comparatively lower downregulation of miR-575 was observed in MDA-T32. Both of these cell lines were used for further studies.
MiR-575 inhibits proliferation of thyroid cancer cells via apoptotic cell death
Next, we attempted to understand the role of miR-575 in thyroid cancer and for that we overexpressed miR-575 in MDA-T32 and MDA-T68 thyroid cancer cells (Figure 2 A). The MTT assay showed significant inhibition of the proliferation rate of the MDA-T32 and MDA-T68 thyroid cancer cells upon miR-575 overexpression (Figure 2 B). Next we performed DAPI staining of the miR-575 mimics transfected MDA-T32 and MDA-T68 cells and we found that miR-575 overexpression causes nuclear fragmentation of both the thyroid cancer cell lines, suggestive of apoptosis (Figure 3 A). Annexin V/PI assay showed that apoptosis was 28.73 and 44.53% in miR-575 mimics transfected as compared to 0.25 and 2.19% in miR-NC transfected MDA-T32 and MDA-T68 transfected thyroid cancer cells (Figure 3 B). The western blot analysis showed the expression of Bax to be enhanced while that of Bcl-2 was suppressed in both the thyroid cancer cells to be downregulated upon miR-575 overexpression (Figure 3 C).
Figure 2
A – Expression of miR-575 in miR-NC and miR-575 mimics transfected MDA-T32 and MDA-T68 cells. B – Cell viability of miR-NC and miR-575 mimics transfected MDA-T32 and MDA-T68 thyroid cancer cells. The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05)
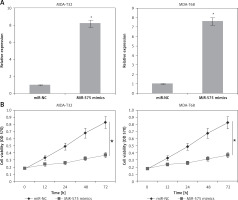
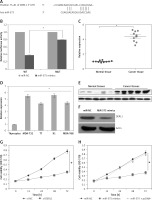
Figure 3
DAPI (A) and Annexin V/PI (B) staining of miR-NC and miR-575 mimics transfected SK-BR-3 and CAMA-1 cells. C – Western blot analysis of miR-NC and miR-575 mimics transfected MDA-T32 and MDA-T68 cells showing the expression of Bax and Bcl-2. The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05)

MiR-575 inhibits migration and invasion of thyroid cancer cells
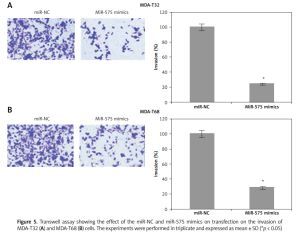
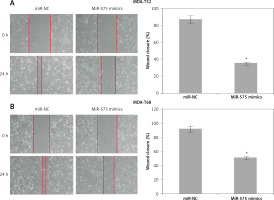
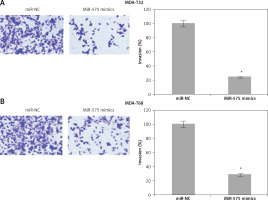
The wound healing assays showed that miR-575 overexpression suppressed the migration of both the MDA-T32 and MDA-T68 cells. The wound closure was found to be around 38 and 51% in miR-575 mimic transfected MDA-T32 and MDA-T68 cells as compared to around 88% and 92% in the miR-NC transfected MDA-T32 and MDA-T68 cells respectively (Figure 4). The transwell assays showed that the invasion of the thyroid cancer cells was also suppressed. The invasion of the MDA-T32 and MDA-T68 cells was suppressed by 79 and 75% relative to the controls respectively (Figure 5). However, we do not rule out the possibility that these inhibitory effects of miR-575 on migration and invasion may also be at least partially due to its growth inhibitory effects.
MiR-575 targets Derlin 1 in thyroid cancer cells
We firstly employed the bioinformatic approaches such as TargetScan to identify the targets of miR-575. TargetScan revealed DERL1 to be the potential target of miR-575 (Figure 6 A). Next, dual luciferase assay also confirmed the interaction between miR-575 and DERL1 (Figure 6 B). We also examined the expression of DERL1 in all the thyroid cancer tissues and the cell lines and the qRT-PCR revealed DERL1 to be aberrantly upregulated in both the thyroid cancer tissues and cell lines (Figures 6 C, D). Additionally, western blot analysis also revealed the DERL1 protein expression to be highly enhanced in cancer tissues (Figure 6 E). However, miR-575 overexpression in the MDA-T68 cells resulted in post-transcriptional suppression of DERL1 (Figure 6 F). We also sought to ascertain whether silencing of DERL1 caused similar effects on the MDA-T68 cells as that of miR-575. We found that DERL1 silencing caused a remarkable decrease in the proliferation of MDA-T68 cells (Figure 6 G). The effects of DERL1 overexpression on the proliferation and colony formation of MDA-T68 thyroid cancer cells overexpressing miR-436 were also investigated. We observed that overexpression of DERL1 in the MDA-T68 thyroid cancer cells overexpressing miR-575 promoted their proliferation, thereby avoiding the tumor suppressive effects of miR-575 (Figure 6 H). These effects were also observed in the case of migration and invasion of the MDA-T68 cells.
Figure 6
A – TargetScan analysis showing DERL1 as the target of miR-575. B – Dual luciferase assay. C – Expression of DERL1 in thyroid cancer and adjacent normal tissues. D – Expression of DERL1 in normal and thyroid cancer cell lines. E – Western blotting showing the expression of DERL1 in normal and thyroid cancer tissues. F – Western blots showing the expression of DERL1 in miR-NC and miR-575 mimic transfected MDA-T68 cells. G – Cell viability of si-NC and si-DERL1 transfected MDA-T68 cells, H – Cell viability of miR-NC, miR-575 mimics and miR-575 + pcDNA-DERL1 transfected MDA-T68 cells. The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05)
Discussion
Thyroid cancer is one of the lethal types of cancers and causes tremendous mortality across the globe. The lack of reliable and efficient therapeutic targets/agents is one of the major obstacles in the treatment of thyroid cancer [13]. This study for the first time reports the tumor suppressive effects of miR-575 in thyroid cancer. The expression of miR-575 was significantly suppressed in the thyroid cancer tissues and cell lines. These findings corroborate previous studies wherein miR-575 has been shown to be aberrantly expressed in non-small lung cancer cells [6]. The ectopic expression of miR-575 caused a decrease in the proliferation rate of the thyroid cancer cells. The ectopic expression of miR-575 also caused induction of apoptosis in the thyroid cancer cells, which was accompanied by an upsurge of Bax and depletion of Bcl-2. These findings are consistent with previous studies wherein miRs have been shown to suppress cancer cell proliferation via induction of apoptosis. For example, miR-34a has been reported to halt the proliferation of neuroblastoma cells via induction of apoptosis [14]. Similarly, miR-708 has been found to induce apoptosis in renal cancer cells [15]. The effects of miR-575 on the migration and invasion of human thyroid cancer cells were also assessed and the results revealed that miR-575 inhibits the migration and invasion of human thyroid cancer cells. In a previous study miR-575 was found to regulate the invasion of lung cancer cells [6]. Bioinformatic analysis and dual luciferase showed that Derlin 1 acts as one of the potent targets of miR-575 in gastric cancer. Derlin 1 (DERL1) was found to be highly overexpressed in thyroid cancer cells and miR-575 overexpression resulted in suppression of DERL1. Silencing of DERL1 also caused inhibition of thyroid cancer proliferation while overexpression of DERL1 could nullify the effects of miR-575 overexpression. These observations are consistent with previous studies wherein DERL1 has been shown to be overexpressed in several cancer types such as non-small lung carcinoma and thyroid carcinoma [16, 17]. DERL1 overexpression has also been shown to promote proliferation of colon cancer [18]. In yet another study DERL1 was shown to promote the invasion of canine mammary adenocarcinomas [19].
In conclusion, miR-575 regulates the proliferation, migration and invasion of thyroid cancer cells by suppressing the expression of DERL1. In conclusion, miR-575 acts a tumor suppressor and may point to a novel therapeutic target in thyroid cancer.