Introduction
The discovery of controlled ovarian hyperstimulation (COH) is essential for artificial reproduction technology (ART). The purpose of COH is to induce multiple follicular developments and to obtain an ideal number of available eggs, which can be used in in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) [1]. The most popular protocol of pituitary down-regulation is the “long-term” gonadotropin releasing hormone agonist (GnRH-a) regimen, in which the pituitary is blocked during the luteal phase of the entry cycle, followed by ovulation stimulation with gonadotropin [2]. In recent years, it has been suggested that prolonged pituitary inhibition may result in a higher pregnancy rate. Although a prolonged program of GnRH-a 3–6 months prior to ovarian stimulation has been reported to provide excellent results in IVF/ICSI [3, 4], a few months of down-regulation before IVF-ET may be unacceptable. Reported studies and our experiences have shown that a new modified prolonged protocol, with down-regulation starting at the beginning of the menstrual cycle, may also improve the clinical outcomes of IVF/ICSI [5].
Controlled ovarian hyperstimulation of IVF is usually performed with follicle stimulating hormone (FSH). However, the appropriate dose of FSH is still debated. To date, the frequent clinical practice of increasing the FSH dosage is expected to obtain a higher number of oocytes, but recent studies have suggested that increasing the dosage of FSH does not lead to a higher pregnancy rate in IVF [6, 7]. A high r-FSH dosage not only increases the cost of IVF, but also might increase the risk of ovarian hyper-stimulation syndrome (OHSS) and the occurrence of aneuploid oocyte [8, 9]. Thus, the mild COH protocol is mainly suggested for normal patients with expectation of a good response.
Although the mild COH protocol has been reported to show good clinical outcomes, few studies have been performed to study the mild COH protocol in a modified prolonged regimen. In the current study, we compared the results of IVF/ICSI in normal patients with good ovarian reserve who underwent COH with a mild starting dosage of FSH with those exposed to a conventional starting dosage in a modified prolonged GnRH-a pituitary down-regulation protocol.
Material and methods
Patients
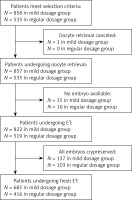
The present study was approved by the Clinical Ethical Committee of Jiangxi Provincial Maternal and Child Health Hospital. In this retrospective study, records of patients who underwent IVF/ICSI-ET treatment from September 2014 to December 2015 in our center were reviewed (Figure 1). Selection of study subjects was according to the following criteria:
Inclusion criteria:
20 ≤ age ≤ 35 years;
infertility history ≥ 1 year;
first time of IVF/ICSI-ET treatment;
ovarian reserve, with antral follicle count ≥ 8 in at least one side of the ovaries;
indication for IVF/ICSI due to one or more of the following: tubal factors, male factors, ovulation disorders (except for polycystic ovary syndrome), and endometriosis;
Exclusion criteria:
one of the following uterine abnormalities: uterine malformations (unicornuate uterus, bicornuate uterus, uterus didelphys, septate uterus), adenomyosis, submucosal myoma, intrauterine adhesions, chromosome karyotype abnormalities in patients;
history of ≥ 3 miscarriages (including miscarriages after biochemical pregnancy).
In addition, patients with the following conditions in contradiction with ART treatments were excluded from IVF/ICSI treatment: uncontrolled diabetes, liver or renal dysfunction without definite clinical diagnosis, history of deep vein thrombosis, history of pulmonary embolism, history of cerebrovascular events, uncontrolled hypertension, heart disease, suspicion of cervical cancer, endometrial cancer, breast cancer or unexplained vaginal bleeding.
Controlled ovarian stimulation and IVF/ICSI procedure
Controlled ovarian hyperstimulation followed a modified prolonged protocol: intramuscular injection of 3.75 mg GnRH-a (long-term acting Diphereline, Beaufour Ipsen, France) was started on day 2–3 of the menstrual cycle. If pituitary down-regulation (endometrial thickness ≤ 5 mm, serum FSH < 5 mIU/ml, luteinizing hormone (LH) < 5 mIU/ml, E2 < 50 pg/ml) was confirmed with transvaginal ultrasound and endocrine examination after 30 days, administration of recombinant human FSH (r-FSH, Gonal-F, Merck Serono, Switzerland) was used to initiate the superovulation cycle. The patients were separated into two groups according to the starting dosage of r-FSH: a mild dosage group with a starting dosage of 75 IU ≤ r-FSH < 150 IU, and a conventional dosage group with a starting dosage of 150 IU ≤ r-FSH ≤ 225 IU. After 5 days, the sizes of follicles and serum hormone levels were monitored to adjust the r-FSH dosage according to follicular growth and development. When at least one follicle with a diameter ≥ 19 mm or 2 follicular diameters ≥ 18 mm were observed, 250 μg of recombinant human choriogonadotropin (HCG, Merck Serono, Switzerland) was administered by subcutaneous injection. Oocyte retrieval was performed 36 h after HCG injection by transvaginal ultrasound-guided puncture of follicles. Luteal support was started on the day of oocyte collection with injection of progesterone (80 mg/day). The oocytes were cultured in vitro for 3 to 6 h followed by in vitro fertilization or intracytoplasmic sperm injection. The embryos with the best quality were selected on the 3rd day after fertilization. If more than 15 oocytes were acquired, the patients would undergo transvaginal ultrasound examination on the day of transplant. In cases with ovarian diameter ≥ 7 cm, reported abdominal distension or bloating, embryos cryopreservation was performed in concern of mild to severe OHSS after transplantation. After transplantation, luteal support was performed with progesterone vaginal sustained-release gel (1 vial/day, 8% Crinone, 90 mg/vial Merck Serono, Switzerland) and dydrogesterone tablets (2 tablets/day, Dapbaston 10 mg/tablets, Solvay Pharma, Netherlands). Biochemical pregnancy was examined at 13 days after transplantation with urine or blood HCG. Clinical pregnancy (defined as detection of gestational sac and heart beat) was determined using ultrasound 1 month after transplantation. Luteal support was maintained until 10 weeks of pregnancy.
Data collection and assessment
Data were collected from clinical records, including demographic and clinical characteristics, COH and IVF/ICSI results such as basal hormone levels on days 2–5 of the menstrual cycle, days of r-FSH administration, total r-FSH dose, serum E2, LH, progesterone levels on HCG day, number of oocytes retrieved, 2PN fertilization rate, good-quality embryo rate, endometrial thickness, total embryo freezing rate, clinical pregnancy rate, implantation rate, miscarriage rate, incidence of mild to severe OHSS, etc. Normal fertilization was confirmed when two pronuclei (2PN) were found in the cytoplasm. The normal fertilization rate refers to the number of fertilized oocytes divided by the total number of all retrieved oocytes. Embryo evaluation was conducted 64–68 h after oocyte fertilization. A good-quality embryo should consist of 7–9 blastomeres with a uniform size, and the fragment proportion should be less than 20%. The high-quality embryo rate refers to the number of high-quality embryos divided by the total number of embryos.
Statistical analysis
SPSS 17.0 (IBM, Armonk, NY, USA) software was used for statistical analysis. Continuous quantitative data were presented as mean ± standard deviation. Differences among groups were compared using Student’s t test. Categorical data were presented as rate (percentage) with differences among groups analyzed using the χ2 test. Statistical analysis was tested on two-sided settings, with p < 0.05 considered as statistically significant.
Results
Demographic and infertility characteristics
A total of 1393 patients who underwent modified prolonged COH and IVF/ICSI treatment were enrolled, including 858 cases of a mild starting dosage of r-FSH and 535 cases of a conventional staring dosage of r-FSH. Demographic and infertility characteristics for both groups are shown in Table I. There was no significant difference in age, infertility duration, body mass index (BMI) or basal hormone levels between the two groups (p > 0.05).
Table I
Demographic and infertility characteristics of patients in mild dosage group and conventional dosage group
Comparison of outcomes of COH and IVF/ICSI between mild dosage group and conventional dosage group
The results of the COH and IVF/ICSI are presented in Table II. There was no significant difference in blood FSH, LH, or E2 levels after pituitary down-regulation. On the HCG day, there was no significant difference in endometrial thickness or progesterone level. Nonetheless, the levels of E2 on the HCG day in the mild dosage group were lower than those in the conventional dosage group (2768.16 ±1453.81 vs. 3161.65 ±1519.75, p < 0.001). Interestingly, although the duration of r-FSH treatment was a little longer in the mild dosage group (11.96 ±2.05 vs. 11.40 ±1.65, p < 0.001), the total r-FSH dosage and the cost of ovarian stimulation were significantly lower than those in the conventional dosage group (1900.95 ±699.40 vs. 2548.36 ±690.89, p < 0.001; 5541.21 ±1237.71 vs. 8696.30 ±1977.09; p < 0.001). Furthermore, compared to the conventional dosage group, the number of retrieved oocytes were also lower in the mild dosage group (14.75 ±5.46 vs. 18.00 ±6.02, p < 0.001), whereas the rates of 2PN fertilized oocytes and good-quality embryos were significantly higher (60.56% vs. 56.59%, p < 0.001; 44.41% vs. 40.05%, p < 0.001).
Table II
Results of COH and IVF/ICSI in mild dosage group and conventional dosage group
Comparison of clinical outcomes between mild dosage group and conventional dosage group
Six hundred and eighty-five patients (1344 embryos transferred) in the mild starting dosage group and 416 patients (820 embryos transferred) in the conventional starting dosage group underwent implantation. As shown in Table III, the implantation rate (55.58% vs. 32.07%, p < 0.001), clinical pregnancy rate (74.16% vs. 63.46%, p < 0.001), and live birth rate (63.94% vs. 53.37%, p < 0.001) were significantly higher in the mild dosage group than in the conventional dosage group. No difference was found in early miscarriage rate, incidence of ectopic pregnancy rate and mild to severe OHSS incidence between these two groups (p > 0.05).
Table III
Results of clinical outcomes in mild dosage group and conventional dosage group
Discussion
Previous studies have reported that patients suffering from endometriosis pretreated with prolonged GnRH-a before IVF obtained a satisfactory pregnancy rate [10, 11]. Recently, several studies have shown that prolonged down-regulation with GnRH-a not only improved the embryo quality, but also increased the pregnancy rate in patients without endometriosis [12, 13]. Furthermore, a study conducted by Ren et al. [5] suggested that COH with a starting dosage of 150–220 IU of gonadotropin after prolonged down-regulation with GnRH-a significantly benefited the live birth rate. Similarly, we also found this superiority by using the same prolonged down-regulation protocol as Ren et al. [5]. Nevertheless, this prolonged protocol is still associated with several problems including increased gonadotropin dosage, higher number of oocytes retrieved and an economic burden, especially in patients with normal ovarian reserve. As is known, COH after pituitary down-regulation is an important procedure to regulate oocyte development before oocyte retrieval. A suitable dosage of gonadotropin in COH might have a beneficial effect on the clinical outcome in IVF. Therefore, we conducted this study to explore whether the reduced r-FSH starting dosage in the COH process could improve the IVF/ICSI outcome in normal ovarian responders.
It has been clearly shown that the ovarian response to FSH depends mainly on the status of the ovarian reserve [14–16], indicating that different status of ovarian reserve treated with a corresponding COH protocol would not improve the IVF/ICSI outcome. A mild FSH dosage was sufficient to maintain a single dominant follicle’s development and ovulation [17, 18]. Previous studies have reported that an FSH concentration above the threshold of 10–30% was enough to stimulate the normal development of follicles, but a further increase of the FSH dosage would lead to excessive stimulation [19]. In our study, compared with the conventional dosage group, the number of oocytes retrieved in the mild dosage group was significantly lower; however, the oocytes in this group showed a higher rate of 2PN fertilized oocytes and good-quality embryos, implying that mild dosage r-FSH may be beneficial to oocyte development and fertilization. This result is confirmed by a previous study by Qiu et al. [20], in which the authors compared the effect of different gonadotropin dosage on oocyte fertilization and embryo development in mice. They demonstrated that the dosage of gonadotropin was clearly negatively correlated with the oocyte fertilization rate and blastocyst rate, suggesting that a higher dosage of gonadotropin might hamper oocyte development and subsequently impair the potential development of the embryo.
It has been reported that a higher dosage of gonadotropin did not improve the implantation rate and pregnancy rate during the IVF/ICSI treatment [21], indicating that high dosage gonadotropin might not be a good choice in the COH protocol after pituitary down-regulation. Similarly, the present study also found that the patients using a mild starting dosage of r-FSH showed a remarkably better implantation rate, pregnancy rate and live birth rate, compared to those using the conventional starting dosage of r-FSH. Interestingly, Out et al. [22] evaluated the efficacy of a lower starting dosage of r-FSH ovarian stimulation in various settings. The authors compared 100 IU and 200 IU r-FSH in patients aged 18–39 years, and found no difference in the pregnancy rate between these two groups. This may be due to the age range of patients in their study, in which they enrolled patients aged from 18 to 39. This might include patients with poor ovarian reserve, especially in patients aged above 36. In our study, all the patients were under 35 years old and were evaluated strictly as having good ovarian reserve. Additionally, a different pituitary down-regulation protocol and population, as well as the agonist chosen in practice may be other influencing factors. Further studies are still needed to confirm whether the mild starting dosage protocol could improve clinical outcomes in general.
Furthermore, the total cost of the procedure is another important factor for consideration among patients undergoing IVF/ICSI [23, 24]. Lower dosage reagents also play a critical role in reducing the financial burden and increasing patients’ comfort. In the current study, it was noted that although the duration of r-FSH treatment was a little longer in the mild dosage group, the total r-FSH dosage and the cost of ovarian stimulation were significantly lower compared to the conventional dosage group. Therefore, mild dosage r-FSH may benefit the patients.
In the present study, there was no difference in the incidence of mild and severe OHSS between the two groups. This might be associated with the method of analysis. In the process of clinical practice, the starting dosage of r-FSH selection was based on the status of ovarian reserve of patients. Generally, a lower starting dosage of r-FSH was often used in the patients with good ovarian reserve, who were much more likely to suffer from OHSS. Therefore, although the total dosage of r-FSH was lower in the mild dosage group than in the conventional dosage group, the incidence of mild and severe of OHSS did not show significant differences between the two groups.
The current study has certain limitations. The study was performed as a retrospective data analysis, and the starting dosage selection for the patients may reflect the difference in clinical characteristics. However, since no significant difference was detected in the baseline measurements, we assumed that the study subjects in the two groups were comparable. In addition, since the IVF/ICSI outcomes may be affected by numerous confounding factors, the results should be analyzed with extreme caution.
In conclusion, the modified prolonged GnRH-a pituitary down-regulation regimen combined with a mild starting dosage of r-FSH improved IVF/ICSI and clinical outcomes, as well as reducing the financial cost in young patients with good ovarian reserve. The efficacy of COH with a mild starting dosage of r-FSH in other patients, such as those with poor ovarian reserve, who are older, and in combination with another pituitary down-regulation protocol, needs to be further studied.