Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
IMMUNOLOGY / BASIC RESEARCH
Moesin expression is correlated with its involvement in patients with Behcet’s disease
1
112 Lab, School of Chemistry and Biological Engineering, University of Science and Technology, Beijing, China
2
Experimental Research Center, China Academy of Traditional Chinese Medicine, Beijing, China
3
Department of Clinical Biochemistry, Chinese PLA General Hospital, Beijing, China
Submission date: 2018-03-06
Final revision date: 2018-08-03
Acceptance date: 2018-08-16
Online publication date: 2020-02-12
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):924-930
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Behcet’s disease (BD) is a rare, chronic autoimmune disorder of unknown etiology. Although the profile of autoantibodies for this disease is not yet completely understood, because of better disease recognition, its prevalence is increasing throughout the world. Among ERM proteins (ezrin/radixin/moesin), moesin is a member of a family which is involved in autoimmune diseases. The aim of this study is to confirm whether moesin is a potential anti-endothelial cell autoantigen (AECA) in Hans Chinese BD patients.
Material and methods:
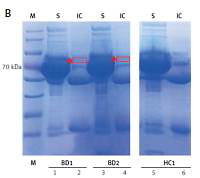
First, a full length recombinant human moesin protein was over-expressed and purified. Second, it was identified by mass spectrometry and then purified moesin was used to perform Western blotting, immunoprecipitation and ELISA with confirmed BD patients. Finally, in vitro cytotoxicity experiments were conducted with anti-moesin antibodies by the resazurin reduction assay method.
Results:
Purified moesin protein was successfully expressed and then its antigenicity was confirmed by Western blotting and immunoprecipitation techniques. Anti-moesin antibodies were detected in approximately one-third (38%) of BD patients by ELISA and the reactivity of BD serum IgG antibodies against moesin was found to be significantly higher than HC (p < 0.0001). Moreover, in order to validate our results, cytotoxicity experiments also confirmed that anti-moesin antibody had a significant inhibitory effect on endothelial cell activity.
Conclusions:
Expression is correlated with the involvement of moesin as an autoantigen in BD pathology, which is a new finding. It might be a new candidate biomarker in the Han Chinese population.
Behcet’s disease (BD) is a rare, chronic autoimmune disorder of unknown etiology. Although the profile of autoantibodies for this disease is not yet completely understood, because of better disease recognition, its prevalence is increasing throughout the world. Among ERM proteins (ezrin/radixin/moesin), moesin is a member of a family which is involved in autoimmune diseases. The aim of this study is to confirm whether moesin is a potential anti-endothelial cell autoantigen (AECA) in Hans Chinese BD patients.
Material and methods:
First, a full length recombinant human moesin protein was over-expressed and purified. Second, it was identified by mass spectrometry and then purified moesin was used to perform Western blotting, immunoprecipitation and ELISA with confirmed BD patients. Finally, in vitro cytotoxicity experiments were conducted with anti-moesin antibodies by the resazurin reduction assay method.
Results:
Purified moesin protein was successfully expressed and then its antigenicity was confirmed by Western blotting and immunoprecipitation techniques. Anti-moesin antibodies were detected in approximately one-third (38%) of BD patients by ELISA and the reactivity of BD serum IgG antibodies against moesin was found to be significantly higher than HC (p < 0.0001). Moreover, in order to validate our results, cytotoxicity experiments also confirmed that anti-moesin antibody had a significant inhibitory effect on endothelial cell activity.
Conclusions:
Expression is correlated with the involvement of moesin as an autoantigen in BD pathology, which is a new finding. It might be a new candidate biomarker in the Han Chinese population.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.