Introduction
Spices are gaining popularity not only in terms of individual consumption, but also in the food industry and medicine. While the incidence of allergies is constantly rising, those caused by the ingestion of herbs and spices are still relatively rare and have not been studied extensively. Therefore, there is little literature data concerning this problem. It is estimated that allergies to spices constitute approximately 2-4% of all food allergies [1]. The allergic potential of spices is dangerous, since they are added to many dishes and products, and consumers may ingest them unknowingly. At particular risk are persons allergic to both birch and mugwort pollen, because of cross-reactivity to proteins similar to the birch allergen Bet v 1 and profilins. These persons as well as individuals who are allergic to celery, very often exhibit an allergic response following the ingestion of spices from the Apiaceae family (previously Umbelliferae), such as anise, coriander, cumin, and fennel. This clinical condition has been described in a study by Wűthrich et al. [2] and termed as “mugwort-celery-spice syndrome”. Later, the term was divided and renamed as “celery-birch-mugwort-spice syndrome” and “celery-carrot-mugwort-spice syndrome”. Allergy to spices is often regarded as a secondary effect accompanying a primary allergy to an airborne allergen [3].
Anise, dill, coriander, and cumin allergens are primarily responsible for type I hypersensitivity. Most of the detected allergens from the Apiaceae family are analogues of Bet v 1 and profilins. The identified anise allergens, such as homologues of Bet v 1 and profilins with a molecular mass of 12-17 kDa have been termed “Pim a 1” and “Pim a 2”, respectively, according to the international allergen nomenclature [4]. Similar allergenic proteins have been discovered in cumin (Cum c 1 and Cum c 2), fennel (Foe v 1 and Foe v 2), and coriander (Cor s 1 and Cor s 2) [5, 6]. Homologues of Bet v 1 and profilins have also been found in another popular spice in the Apiaceae family, i.e. parsley (Petroselinum crispum), and named “Pet c 1” (17.5 kDa) and “Pet c 2” (14 kDa), respectively [3]. The caraway analogs of Bet v 1 and profilins are not yet identified. However, some physicians reported anaphylactic shock caused by spices with caraway [7]. The risk for people allergic to birch pollen, mugwort, or celery is especially significant.
Other allergens include proteins with a molecular mass of 12.9-13.7 kDa detected in anise [8], IgE-binding proteins with a molecular mass of 48, 42, 39, 37, 34, 33, and 20 kDa found in anise extracts, and with a molecular mass of 54, 42, 38, 31, and 20 kDa in cumin extracts [9].
In 33% of patients diagnosed with allergy to spices, but not to pollens (birch, mugwort) or celery, IgE was not bound by any of the spice proteins. It is therefore possible that those particular clinical reactions may be caused by other types of hypersensitivity, such as II, III, or IV. Because spices contain highly reactive substances, the symptoms may be equally classified as food intolerance [6].
The aim of our research was to verify the extracts of popular spices, anise and caraway, for the presence of major pan-allergens, such as Bet v 1 analogues and profilins. Secondly, we analyzed the prevalence of reactions towards these pan-allergens among patients sensitive to spices. Finally, we tried to identify some of the non-typical allergenic proteins in anise and caraway.
Material and methods
Preparation of spice extracts
Carum carvi L. (caraway) and Pimpinella anisum L. (anise) were purchased from a local spice shop in Poland. The spices were grounded to fine powder (700 µm particle size) in a laboratory mill (Bionovo, PL) and subjected to extraction twice. 1 γ of each spice was immersed in a 5 ml of (Tris)-glycine (T-G) extraction buffer, composed of 0.05 M Tris, 0.33 M glycine, protease inhibitors (Sigma-Aldrich, St. Louis, Missouri, USA), and 3 mM sodium azide (P<sup>o</sup>Ch, PL). Then, they were incubated on a vortex mixer for 1 h, and centrifuged at 3500 rpm over 10 min (Sigma 2-16P centrifuge, Polygen, Wroclaw, PL). Subsequently, the supernatant was collected, while the sediment was re-immersed in 5 ml of extraction buffer and incubated on a vortex mixer for 1 h and centrifuged. The final supernatant was collected to test tubes and subjected to dialysis, using a membrane with a MWCO of 6-8 kDa (Spectrum Laboratories, Miami, Florida, USA) against extraction buffer (pH = 8.3). The dialysates were lyophilized and stored at -20°C until analysis [10]. Positive control preparation was made in the same way from 1 γ of homogenized peach pulp and peel suspended in 5 ml of extraction buffer. Protein concentration was determined with Pierce method, using BCA protein assay kit (Thermoscientific, Rockford, Illinois, USA) with bovine serum albumin (BSA) as a standard.
Electrophoresis of proteins in polyacrylamide gel
SDS-PAGE (15% running gel, 4% stacking gel) was carried out by the Laemmli [11] method in T-G buffer at pH = 8.3 (192 mmol/l glycine, 25 mmol/l Tris, and 0.1% SDS), using a mini vertical electrophoresis apparatus (Kucharczyk, Warsaw, Poland). All reagents used in electrophoresis were purchased from Sigma-Aldrich (St. Louis, MO, USA). Samples were mixed in a 1 : 1 ratio, with sample buffer composed of 125 mM Tris-HCl (pH = 6.8), 2% SDS, 10% glycerol, 5% β-mercaptoethanol, and 0.002% bromophenol blue. Subsequently, the samples were denatured by heating at 95°C for 5 min and loaded onto gel (20 µg protein/well). A pre-stained protein molecular weight marker (Thermo Scientific, USA) was used for the mass with a range from 20 to 120 kDa, and an unstained protein molecular weight marker (Fermentas, LTU) was used for the weight with range from 14.4 to 116 kDa. Electrophoresis was conducted at a constant voltage of 90 V for the stacking gel, and at 135 V after the samples entered the running gel.
Protein blotting
Three parallel blot transfers were performed, in which the extracts from anise and caraway were analyzed with the use of sera of patients sensitive to spices, and antibodies against Bet v 1 and profilins.
Electrophoretic transfer of proteins from gel to nitrocellulose membrane (Sigma-Aldrich, USA) was carried out using a Minitrans apparatus (Kucharczyk, Warsaw, Poland) in T-G buffer, pH = 8.3 (25 mM Tris, 192 mM glycine, 20% methanol) at 20 V overnight. Blocking was conducted for 2 h, using PBS-T buffer solution (PBS pH = 7.4 with 0.1% Tween 20 (Sigma-Aldrich, USA)) with 3% non-fat dry milk, and the membranes were washed with PBS-T buffer solution (3 × 5 min).
For the analysis of spice extracts’ reaction with human sera, the membranes were incubated overnight at 4°C, gently mixed, with the sera of patients allergic to spices diluted in 1 : 5 ratio. The applied secondary antibodies were mouse anti-human IgE antibodies (Sigma-Aldrich, USA) diluted in 1 : 5000 ratio. The negative controls were blots obtained for sera of patients without a history of allergies.
For the determination of Bet v 1 analogues, the membranes were incubated for 2 h with mouse sera containing monoclonal anti-Bet v 1 antibodies (Dendritics, FR) in 1 : 500 dilution. The secondary antibodies were goat anti-mouse IgG antibodies (1 : 5000 ratio) labeled with alkaline phosphatase (Sigma-Aldrich, USA). The negative controls were blots obtained without primary antibodies, and the positive control was peach extract.
For the determination of profilin analogues, the membranes were incubated for 2 h with rabbit polyclonal anti-Bet v 2 antibodies (Cusabio, USA) diluted 1 : 1000. The secondary antibodies used were goat anti-rabbit IgG antibodies (1 : 5000) labeled with alkaline phosphatase (Sigma-Aldrich, USA). The negative controls were blots obtained without the primary antibodies, and the positive control was peach extract.
Following incubation, the blots were washed with PBS-T (3 × 5 min) and incubated for 1 h with an appropriate solution of respective secondary antibodies labeled with alkaline phosphatase diluted with 1 : 5000 ratio (monoclonal anti-human IgE, goat anti-mouse antibodies, or goat anti-rabbit antibodies). Following incubation, the membrane was washed with PBS-T buffer (3 × 5 min), and then developed in a BCIP/NBT (Sigma-Aldrich, USA) substrate solution for alkaline phosphatase until the appearance of bands with satisfactory intensity (15 min).
All the obtained blot electrophoregrams were processed using Gelscan software (Kucharczyk, Warsaw, Poland).
LC-MS/MS protein analysis
Protein analysis using LC-MS/MS was conducted in the biochemical laboratory of the Biocentrum company. Samples were prepared for analysis by destaining, reduction, alkylation, trypsin digestion, and peptide extraction from gel.
Peptides were separated using an UltiMate 3000RS LCnanoSystem chromatograph (Dionex) and subsequently analyzed online, using a MicrOTOF-QII spectrometer (Bruker, Germany) equipped with an Apollo Source ESI nano-sprayer. A C18 cartridge (Acclaim PepMap Nano trap Column) was used; the mobile phase included 2% acetonitrile (ACN) with 0.05% trifluoroacetic acid (TFA). Proper separation was carried out on a 15 cm × 75 µm RP column (Acclaim PepMap 75 µm 100, A° Nano Series TM Column), using a gradient of 2-40% ACN with 0.05% TFA for 30 min. The spectrometer was set to data-dependent acquisition (DDA) in the MS/MS mode with fragmentation of the most intensive ionic precursors.
Preliminary data processing was conducted using Data Analysis 4.0 software (Bruker, Germany) to produce files in Mascot generic format (.mgf). The data were used to query SwissProt database with a taxonomic restriction “Green Plants” and to search the National Center for Biotechnology Information (NCBI) database with the “Apiaceae” restriction. The Mascot server search algorithm (v.3.0, Matrix Science, London, UK) was applied with the following search parameters: enzyme specificity – trypsin, number of allowed missed cleavage sites – 1, fixed modification – carbamidomethylation (C), variable modification – oxidation (M), mass range – unlimited, precursor mass tolerance – ±20 ppm, fragment ion tolerance – ± 0.05 Da.
Protein sequence identification was conducted with the Mascot software, and the SwissProt and NCBI databases (taxonomy of the selected organism and Homo sapiens – to detect any potential contamination).
Results
In order to identify some of the allergenic proteins of anise and caraway, Bet v 1 analogues and profilins, we conducted immunoblotting of the proteins extracted from the spices with anti-Bet v 1 and anti-profilin antibodies.
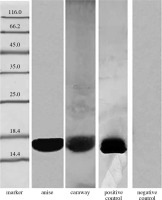
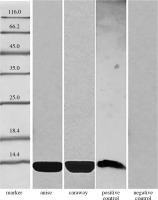
The presence of Bet v 1 analogues in anise and caraway extracts was analyzed by immunoblotting with anti-Bet v 1 monoclonal antibodies (Fig. 1). The results confirmed the presence of Bet v 1 analogues in the studied spices, anise and caraway, exhibiting strong antibody binding with OD = 178654 and 70912, respectively.
Our tests for detection of profilin analogues involved polyclonal antibodies raised against birch profilin 1 with a mass of 15 kDa. As can be seen in Figure 2, a strong reaction of the rabbit serum was induced by the caraway protein (OD = 377344) and by the anise protein (OD = 102816).
Finally, 16 human sera of patients allergic to birch pollen, mugwort pollen, and/or celery, who were also hypersensitive to spices were used in blotting with the same spice extracts. In the case of 6 sera, anise and caraway proteins with a molecular mass of 17 kDa exhibited strong antibody binding. Five of the studied sera reacted with proteins from anise and caraway, like rabbit antibodies against profilin, binding a 15 kDa protein. The remaining sera did not exhibit binding to any proteins extracted from anise or caraway.
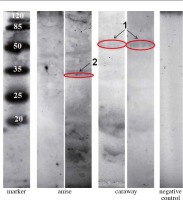
From the sera reacting with Bet v 1 protein, two exhibited additionally a non-typical binding to other protein fractions. These were named “serum 1” and “serum 2” and were subjected for further extended analysis.
The results of Western blot analysis using patient sera 1 and 2 are given in Figure 3, and Tables 1 and 2. The molecular masses of the main proteins binding to human IgE were in the range of 55.7-15.6 kDa for caraway extracts and 57.1-34.2 kDa for anise extracts. Intensive reactions with the sera were found for caraway proteins with the following molecular masses: 15.6 kDa (OD = 223788), 55.7 kDa (OD = 158320), and 56 kDa (OD = 114648), and for anise proteins with the following masses: 57.1 kDa (OD = 239888), 49.6 kDa (OD = 144060), 35.9 kDa (OD = 115920), and 34.2 kDa (OD = 152292).
Table 1
Molecular masses of caraway and anise proteins exhibiting immunoreactivity with the sera of allergic patients (serum 1)
Table 2
Molecular masses of caraway and anise proteins exhibiting immunoreactivity with the sera of allergic patients (serum 2)
| Anise | Caraway | ||||
|---|---|---|---|---|---|
| Relative migration (%) | Molecular weight | I.O.D. | Relative migration (%) | Molecular weight | I.O.D. |
| 25.26 | 57.07 | 239888 | 26.12 | 55.71 | 158320 |
| 30.28 | 49.65 | 105336 | 69.20 | 16.85 | 103520 |
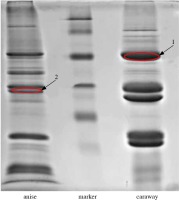
In order to determine the amino acid sequence of allergenic proteins, LC-MS/MS analysis was performed for two immunoblot bands (marked in red in Figure 3 and 4) with a mass of 56-55.7 kDa for caraway (band “1”) that was bound by IgE from two patients and present in both immunoblots, and 34.2 kDa for anise (band “2”), which was strongly bound by IgE from one patient, present in only one immunoblot.
Fig. 4
Allergenic proteins selected for LC-MS/MS analysis, reacting with the sera of individuals allergic to spices
Supplementary Table presents the results of LC-MS/MS analysis and includes proteins identified with high confidence as ascertained by high scores, sequence coverage percentages (SC%), and numbers of peptides identified. Identification was deemed reliable if it was conducted using at least two sequenced peptides with a score of over 100, except for endoribonuclease Dicer homologue 3a, which was identified based on 1 unique peptide with a high degree of certainty.
The results for the bands designated as “1” and “2” depended on the queried database (“Apiaceae” or “Green plants”).
Band 1 was primarily identified as elongation factor 1 α in a search within the “Apiaceae” category.
Band 2 was primarily identified as glyceraldehyde-3-phosphate dehydrogenase in a search within the “Apiaceae” category.
Queries involving larger databases, such as “Green Plants”, may provide additional valuable information if the narrower datasets are incomplete.
Discussion
Proteins homologous to Bet v 1, occurring in different fruits and vegetables, are major allergens in pollen and food allergies across Northern Europe. In the present study, mouse monoclonal anti-Bet v 1 antibodies were used, which are specific for birch (Betula alba) pollen, and which were found by Lebecque et al. [12] to exhibit cross-reactivity to plant allergens homologous to Bet v 1, including Aln γ 1 (alder pollen), nCor a 1 (hazel pollen), Cor a 1 (hazelnut), and nMal d 1 (apple). Bet v 1 homologues were also observed in other spices belonging to the botanical family Apiaceae, such as parsley (Petroselinum crispum), celery (Apium graveolens), and carrot (Daucus carota). Those allergens were named as “Pet c 1” (17.5 kDa), “Api γ 1”, and “Dau c 1”, respectively [4]. Bet v 1 homologues have been detected in the aerial parts of young fennel, but not in its seeds [1].
The presence of the Bet v 1 protein in anise, which was detected in this study, is consistent with the report of Jensen-Jarolim et al. [6], who found a 17 kDa homologue of Bet v 1 in this spice as well as in cumin, fennel, and coriander; the allergens were termed as “Pim a 1”, “Cum c 1”, “Foe v 1”, and “Cor s 1”, respectively [4]. No literature data concerning the presence of Bet v 1 homologues in caraway was found.
Profilins are highly cross-reactive allergens binding to IgE in almost 20% of patients allergic to birch pollen and plant-based food [13]. According to the literature data [5, 6], the largest number of profilin homologues have been detected in spices from the Apiaceae family, such as anise, cumin, fennel, and coriander; these allergens have been termed as “Pim a 2”, “Cum c 2”, “Foe v 2”, and “Cor s 2”, respectively [4]. Similarly, as in the present work, those profilins were identified by means of rabbit anti-profilin antibodies and immunoblotting. Additionally, the presence of profilins was supported by immunoblotting inhibition with the recombinant antigen Bet v 2. This is consistent with the present study and confirms the presence of profilin homologues in anise. The profilin homologues that have been previously identified in other spices belonging to this family include Api γ 4, Dau c 4, and Pet c 2, in celery, carrot, and parsley, respectively [14].
Allergens similar to profilins have also been identified in saffron pollen and stamins (15.5 kDa saffron allergen has been termed as “Cro s 2”) [14, 15]. The expression of profilins within a given plant type may vary, for instance, profilin homologues have been found in the aerial parts of young fennel, but not in fennel seeds [1]. A profilin analogue has also been detected in onion (All c 4) [14].
There is no data on profilins in caraway, but since it belongs to the same plant family as cumin (Apiaceae), the presence of this allergen in caraway seems highly probable, which was confirmed in this work.
Next stage was an identification of the most allergenic fraction of spice proteins that reacted with sera of patients allergic to birch pollen, mugwort pollen, and/or celery, with hypersensitivity to spices.
The strong antibody binding of caraway proteins with a molecular mass of 17 kDa by 6 human sera confirmed that these patients were sensitive to the Bet v 1 allergen present in the spices. From binding a 15 kDa protein in anise and caraway by 5 human sera, we could conclude that these patients were sensitive to the profilin protein fraction.
The results of Western blot analysis using patient sera 1 and 2 for caraway proteins showed that both sera probably bound the same two proteins with a molecular mass of 55.7-56 kDa and 16.9-15.6 kDa. The smaller protein could be Bet v 1. The other band with a molecular mass of 56 kDa was analyzed using LC-MS/MS. The difference in masses may be due to various parameters of computer processing of the blots.
The detected 57 kDa anise protein may correspond to the 60 kDa molecule identified by Jensen-Jarolim et al. [6], which along with Bet v 1 and Bet v 2, represents the group of cross-reacting allergens in celery-birch-mugwort-spice syndrome. The immunoblotting analysis conducted by Gázquez García et al. [8] involved the serum of person allergic to anise liqueur and led to the detection of allergenic anise and vetch proteins ranging from 12.9 to 13.7 kDa, and cumin proteins ranging from 15 to 17.5 kDa. Interestingly, IgE binding was not found in the case of anise liqueur. Our immunoblotting results did not find any reactions for anise proteins in this mass range, which may be explained by the type of serum used in the study of Gázquez García et al. [8]. In this study, in contrast to that used in the present work, the serum was derived from a person allergic to anise proteins, with the highest titre to vetch, cumin, and anise.
The allergenic properties of anise were confirmed by another study Garcia-González et al. [9], reporting a case of woman with occupational allergy to anise. In a prick skin tests, the woman exhibited immediate reactivity to caraway, anise, coriander, fennel, and dill. Immunoblotting showed that the patient’s IgE bound to anise proteins with a molecular mass of 48, 42, 39, 37, 34, 33, and 20 kDa, and caraway proteins with a molecular mass of 54, 42, 38, 31, and 20 kDa. Our blots revealed the following protein bands similar to those above-mentioned: 49.7, 49.5, 42.5, 39.3, 35.9, 34.2 kDa (anise), and 55.7 kDa (caraway).
LC-MS/MS analysis identified sequence of one caraway and anise protein allergenic for patients sensitized to spices, which was recognized as elongation factor α and glyceraldehydes-3-phosphate dehydrogenase, respectively. Some information about these compounds has been found in several databases.
Eukaryotic elongation factor α (eEF-1A) from carrot, with a length of 447 aa, occurs in a high quantity, accounting for 1-3% of total soluble cellular proteins. Moreover, eEF-1A has been found to fulfill a variety of functions, playing a role in protein synthesis, binding and packing of actin, activation of phosphatidylinositol 4-kinase, binding and cleavage of microtubules as well as participation in ubiquitin-mediated degradation of N-acetylated proteins. Even though the amino acid sequence of all eEF-1A is highly conservative, it is variable in terms of the presence and location of post-translational modifications [16]. According to the Allergome database [14], examples of allergenic plant proteins of this type include a latex allergen from Siphonia brasiliensis, Hev β 1 (138 aa) [17]. Elongation factor 1 β is also a fungal allergen found in Penicillium citrinum, Pen c 24 (228 aa) [17].
Carrot glyceraldehyde 3-phosphate dehydrogenase (GAPDH), with a length of 337 aa, is a key enzyme (oxidoreductase) participating in glycolysis, catalyzing the conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate. This protein plays an important role in maintaining cellular ATP and carbohydrate metabolism [17, 18]. The UniProt database [17] lists Blo t (416 aa) from the mite Blomia tropicalis as an allergenic glyceraldehyde 3-phosphate dehydrogenase. In turn, according to the Allergome database [14], allergenic proteins of this kind mostly include airborne insect allergens and airborne allergens from fungi in Alternaria (Alt a 10), Beauveria (Bea β Ald), and Cladosporium (Cla h 10) families. Finally, the Allergen database [4] lists Tri 34, an airborne allergen from wheat (Triticum aestivum) as a plant allergen of this type.