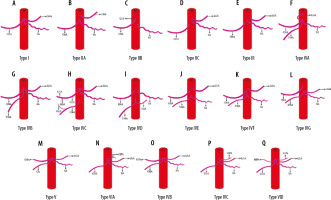
Introduction
The celiac artery (CA) is the main artery in the upper abdomen, supplying the major organs such as the lower end of the oesophagus, stomach, part of the duodenum, spleen, pancreas, and liver [1]. The CA is the first ventral branch of the abdominal aorta (AA), and it commonly shows a trifurcation branching pattern: left gastric artery (LGA), common hepatic artery (CHA), and splenic artery (SA). Occasionally other branching patterns like bifurcation, quadrifurcation, pentafurcation, and hexafurcation can also be seen. In these patterns, the additional branches that arise from the CA include the superior mesenteric artery (SMA), inferior phrenic artery (IPA), gastroduodenal artery (GDA), middle colic artery (MCA), accessory hepatic artery, suprarenal arteries, retro-portal artery, aberrant bronchial artery, and dorsal pancreatic artery [2]. The CA was first described by Haller as the tripus Halleri in 1756. CA variations were first classified by Lipshutz (1917) into 4 types based on the origins of the CHA, SA, and LGA. Various authors like Adachi et al. (1928) and Michel et al. (1955) classified CA into 6 types by incorporating the origin of the superior mesenteric artery (SMA) with CA. Uflacker (1997) classified CA into 8 types by adding the origin of SMA and MCA with CA and no CA [3-6]. Song et al. (2010) classified CA into 15 types, which included the above-mentioned arteries [7]. Panagouli (2013) classified CA into 10 types, in which he added the inferior mesenteric artery (IMA), along with the above-mentioned arteries. In this study, we have proposed a new classification to define the CA variations on the basis of embryology and correlation with routine computed tomography angiography (CTA) studies on 1050 patients. Knowledge of the CA branching pattern, its origin, and course is not only limited to anatomy but also plays a significant role clinically. It may be the source of pathological conditions like celiac compression syndrome and gastrointestinal bleeding. Patients should undergo diagnostic angiography prior to operative procedures and interventional procedures to identify the anatomical variations because knowledge of vascular anatomy is obligatory before planning treatment [8]. Because the classifications of CA variations available to date do not encompass all variants of CA branching patterns, we have proposed a new classification that incorporates most of the variants.
Material and methods
We retrospectively assessed the celiac trunk variations in patients who came to our department for diagnostic CT angiography for liver and renal donor workup. The patients’ data were acquired from the Picture Archiving and Communication System of our institution. The study was approved by the Institutional Human Ethics Committee (Ref. No. 002/SBMC/IHEC/2019/1253) and a waiver of the consent form was obtained. We collected and reviewed the CT angiogram images performed between June 2018 and July 2020. Multiphasic helical CT was done on a 128-slice CT scanner (Siemens Perspective, Shanghai, China) and a 32-slice CT scanner (Siemens SOMATOM) for the study. We initially included all the 1113 CT studies done during the period. The 63 CT studies showing major acquired vascular pathologies and motion artefacts were excluded from the study. We finally included 1050 cases, which comprised 482 males and 568 females. The region of interest extended from just above the diaphragm to the level of the iliac vessel bifurcation. The scanning parameters were as follows: slice thickness – 5.0 mm; slice direction – craniocaudal, (rotation time: 0.6 seconds) detector configuration with 64 × 0.6 mm, 150 mAs; 130 kVp; pitch – 0.8. The images obtained were later reconstructed into thin axial images of 1.0 mm thickness. Non-ionic iodinated contrast material was injected through a sterile 18-gauge cannula inserted into the antecubital vein. The dose range was 100-120 ml and the rate of injection was 5-6 ml per second. The iodine concentration used was 350 mg/ml. Bolus triggering was used with ROI placed in the abdominal aorta, and the images were acquired with a delay of 3 seconds.
The data from PACS was transferred to the workstation for multiplanar reformation, volume rendering, and 3D reconstruction with maximum intensity projection (MIP). The images obtained were independently reviewed by 2 experienced radiologists who have 10 and 12 years’ experience, respectively. We analysed the origin and branching pattern of celiac trunk including LGA, SA, CHA, SMA, and IPA. Initially, images were reconstructed in the axial section at the level of celiac trunk origin. Next, bilateral IPAs were assessed after adjusting the MIP. Later CA and SMA origin and branching patterns were assessed. Overall origin and branching of the above-mentioned arteries were analysed after 3D reconstruction and volume rendering also.
Results
We evaluated CTA studies of 1050 patients, of whom 46% were male and 54% were female. A normal trifurcation pattern was observed in 39% of cases. Celio-phrenic trunk was the second most common pattern, with CA + LIPA being the commonest subtype. Other variations noted in the study and their incidences are listed in Table 1. We attempted to propose a new classification based on embryology, which comprises 6 main types and their subtypes (Table 1). A few subtypes can co-exist, which are mentioned in Table 2. The comparison of the origin and branching pattern of CA on cadaveric and CTA studies is given in detail in Table 3 [4,9-22].
Table 1
Frequency of celiac trunk variations as proposed in our new classification based on embryology in correlation with computed tomography angiography
Table 2
Coexisting types with their frequencies
| Coexisting types | Prevalence | Prevalence % |
|---|---|---|
| 1 + 4c | 17 | 22.60 |
| 4c + 6a | 11 | 14.60 |
| 4c + 6b | 7 | 9.30 |
| 4c + 6c | 13 | 17.30 |
| 4c + 6d | 9 | 12.00 |
| 4c + others | 5 | 6.60 |
| 2c + 4b | 10 | 13.30 |
| 2b + 4e | 1 | 1.35 |
| 2a + 4c | 1 | 1.35 |
| 3 + 4c | 1 | 1.35 |
| Total | 74 | 100.00 |
Table 3
Comparison of the origin and branching pattern of computed tomography based on Michel’s Classification (1951) – cadaveric studies and computed tomography angiography (CTA) studies
| Authors name and year | I | II | III | IV | V | VI | Others | No. of cases | |
|---|---|---|---|---|---|---|---|---|---|
| Cadaveric studies | |||||||||
| Adachi (1928) [4] | 86 | 8 | 1 | 0 | 3 | 1.5 | 0.5 | 252 | |
| Wadhwa and Soni (2011) [9] | 93.2 | 6.7 | 0 | 0 | 0 | 0 | 0 | 30 | |
| Prakash et al. (2012) [10] | 86 | 8 | 0 | 2 | 0 | 0 | 4 | 50 | |
| Chitra (2010) [10] | 80 | 2 | 0 | 0 | 4 | 0 | 4 | 50 | |
| Dsouza et al. (2012) [11] | 90 | 0 | 0 | 0 | 0 | 0 | 10 | 20 | |
| Pusphlata (2006) [10] | 70 | 0 | 0 | 0 | 0 | 0 | 26 | 50 | |
| Sawant (2013) [10] | 86 | 2 | 0 | 0 | 0 | 0 | 12 | 50 | |
| Mburu et al. (2010) [10] | 62 | 13.1 | 0 | 0 | 0 | 0 | 24.9 | 123 | |
| Petrella et al. (2007) [12] | 82 | 2.2 | 0 | 0 | 3.4 | 0 | 12.3 | 89 | |
| Chaitanya et al. (2012) [13] | 98 | 0 | 0 | 0 | 0 | 2 | 0 | 50 | |
| Malnar (2010) [10] | 92 | 0 | 4 | 0 | 0 | 2.5 | 1.5 | 90 | |
| Rossi (1904) [14] | 87 | 10.9 | 0 | 0 | 1.8 | 1.9 | 0 | 102 | |
| Descomps (1910) [9] | 56 | 32 | 0 | 8 | 0 | 0 | 12 | 50 | |
| Picquand (1910) [9] | 74 | 10 | 0 | 6 | 8 | 0 | 4 | 50 | |
| Lipschutz (1917) [3] | 49.3 | 25.3 | 0 | 3.6 | 14 | 0 | 0 | 83 | |
| Eaton (1917) [9] | 67.2 | 22.3 | 0 | 4.8 | 0 | 0 | 5 | 206 | |
| Poynter (1922) [15] | 89 | 9 | 0 | 0 | 2 | 0 | 0 | 160 | |
| Rio Bronco (1912) [9] | 60 | 30 | 0 | 6 | 4 | 2 | 0 | 50 | |
| Michels (1942) [7] | 82 | 4 | 0 | 2 | 4 | 0 | 10 | 50 | |
| Tsukamato (1929) [16] | 82 | 2.6 | 0 | 0 | 1.8 | 0 | 13.2 | 100 | |
| Shoumura (1991) [10] | 90.2 | 1 | 0 | 0 | 0 | 0 | 7.8 | 450 | |
| Vandamme (1985) [17] | 86.6 | 6.4 | 0 | 0 | 0 | 0 | 7 | 156 | |
| Imakoshi (1949) [10] | 90.7 | 3.7 | 0.9 | 0 | 0.9 | 0.9 | 2.8 | 107 | |
| CTA studies | |||||||||
| Song et al. (2010) [7] | 89 | 4.4 | 0.7 | 0.2 | 0.2 | 1.06 | 4.4 | 5002 | |
| Ugurel (2010) [10] | 89 | 3 | 1 | 1 | 4 | 0 | 2 | 100 | |
| Koops (2004) [7] | 95.9 | 0 | 0 | 0 | 3 | 0 | 1.1 | 604 | |
| Matsuki (2004) [18] | 89 | 5.5 | 0 | 2.7 | 2.7 | 0 | 0 | 36 | |
| Ferrari (2007) [19] | 85 | 3.3 | 0 | 0 | 8.3 | 1.7 | 0.4 | 60 | |
| Natsume et al. (2011) [20] | 90.8 | 4.5 | 0 | 0 | 1.1 | 0 | 4 | 175 | |
| Sureka et al. (2013) [17] | 91.1 | 2.8 | 0.16 | 0 | 1.4 | 0.66 | 4 | 600 | |
| Yi wang et al. (2014) [21] | 89.8 | 0.27 | 1.73 | 0.13 | 0.53 | 3.4 | 4.14 | 1500 | |
| Araujoif et al. (2018) [22] | 43 | 47 | 2 | 0 | 5 | 0 | 5 | 100 | |
| Our study | 38.9 | 2.66 | 0.38 | 0 | 3.4 | 0.09 | 54.5 | 1050 | |
Based on the pattern of variations seen in the branching pattern of the CA in the present study, we propose the following embryological basis for CA variations (Figure 1, Table 4).
Table 4
Embryological basis of celiac trunk variations
Discussion
Variations in the branching pattern of CA have an embryological basis. During the first trimester, a major part of vascular development occurs on the 23rd and 36th days of development [23,24]. During the third week of deve-lopment of the heart, the paired dorsal aortae developing along with it supply the numerous bilateral ventral segmental arteries, which in turn supply the abdominal organs. The dorsal aortae fuse during the fourth and fifth weeks of intrauterine life, to form the abdominal aorta (AA), along with the fusion of the dorsal mesentery during the development of intestine. Each dorsal aorta gives numerous ventral splanchnic branches which fuse during this time of intrauterine life, give rise to several ventral branches which runs along the dorsal mesentery and fuses longitudinally in midline to form ventral anastomosis [25]. Along with the formation of ventral anastomosis, regression of various splanchnic branches occurs, with persistence of 3 major branches, namely the CA, SMA, and inferior mesenteric artery, which supply the foregut, midgut, and hindgut, respectively [24]. This process occurs in a cranial to caudal direction. During embryonic development, 9 lateral splanchnic arteries are present on either side of the AA; these arteries in turn supply mesonephrons, metanephrons, gonads, and the adrenal glands, which are derived from intermediate mesenchyme of the mesonephric ridge. These branches are known as the inferior phrenic (first lateral branch of AA), adrenal, renal, and gonadal arteries. Persistence, regression, and fusion of these lateral splanchnic branches give rise to various anatomical variations [26].
The development of IPA can be explained by the ladder theory, which was proposed by Felix [27]. He stated that the development of IPA occurs from a cranial group of lateral mesonephric arteries. Variations occurring in the renal, middle suprarenal, and gonadal arteries also contribute to IPA development along with the above-mentioned mesonephric arteries. However, the celiac origin of IPA cannot be explained by this theory. Abdominal origin of IPA can be explained by the theory proposed by Isogai et al. [28], who suggested that on the 14th day of embryonic life, the cranial part of the adrenal arteries form IPA, and these adrenal arteries are derived from a few branches of the abdominal aorta and/or gonadal arteries. On the 14th-15th day it reaches the diaphragm. The definitive branching pattern is established by day 15 [29].
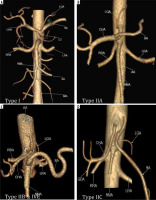
In our study conducted in 1050 patients, normal trifurcation (Type I) was observed in 38.85% of patients (Figure 2A, 3A), while Song et al. found normal anatomy of CA in 89.1%. Type IIa was seen in 2.66% compared to 4.42% in his study (Figure 2B, 3B). Type IIb was observed in 0.09% in our study while Song et al. found this variant in 0.16% of cases (Figure 2C, 3C).
Figure 3
Computed tomography angiography images showing: A) normal trifurcation of celiac artery (Type I), (B) hepatosplenic trunk with left gastric artery (LGA) from the aorta (Type IIA), (C) hepatogastric trunk (Type IIb) with co-existing splenomesenteric trunk of (Type IVe), (D) gastrosplenic trunk with common hepatic artery (CHA) from the aorta (Type IIC)
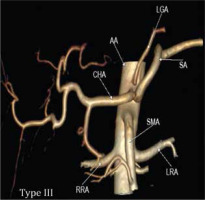
Type IIc was observed in 3.43% in our study while Song et al. found this variant in 0.22% of cases (Figure 2D and 3C). Type III variant, in which the celiac trunk is absent and each branch originate independently from the aorta, was found in 0.57% in our study and in 0.22% in the study by Song et al. (Figure 2E and 4). Type IVa was seen in 0.19% of cases, unlike Song et al. who found this variant in 1.06% of cases (Figure 2F and 5A). Type IVb was encountered in 0.95% of cases while Song et al. found it in 0.24% of cases (Figure 2G and 5B). The frequency of Type IVc was 6.09% in our study but was not reported by Song et al. (Figure 2H and 5C). Type IVe was observed in 0.09% in our study while Song et al. found this variant in 0.16% of cases (Figure 2J and 3C). The frequency of Type IVf was 0.95% while in the study by Song et al. it was 2.64% (Figure 2K and 5D). Type IVg was not found in our study, but Song et al. found it in 0.06% of cases (Figure 2L). We did not find Type IVd in our study, and this was also not reported by Song et al. However, 2 cases were reported by Ray et al. (Figure 2I) [7,30]. Type V was also not found in our study but has been reported by Uflacker et al. in their cadaveric study (Figure 2M) [6]. We compared the prevalence of Type VIa to VId with a study conducted by Ramazan et al. on variations of the inferior phrenic artery in 600 patients. Our study showed a prevalence of 11.33% for Type VIa, which is the common inferior phrenic artery (CIPA) from the CA (Figure 2N and 6A), while Song et al. found about 13.4% of cases with celiaco-phrenic variation. We found 6.09% of cases belonging to Type VIb (Figure 2O and 6B), while Song et al. saw this variation in 25.0% of cases. Type VIc was seen in 18.09% cases in our study, while Song et al. found this variant in 42.2% of cases (Figure 2P and 6C). The frequency of Type VId has not been reported in the literature as per our knowledge, but the prevalence in our study was 16.09% (Figure 2Q and 6D). The frequency of other variants in our study was 2.19% [31].
Figure 5
Computed tomography angiography images showing: A) celiomesenteric trunk (Type IVA) from the aorta, B) hepatomesenteric trunk (Type IVB) with left gastric artery (LGA) and splenic artery (SA) arising separately from the aorta, C) right hepatomesenteric trunk (Type IVC) from the aorta with left hepatic artery (LHA) from common hepatic artery (CHA), D) hepatosplenomesenteric trunk (Type IVF) with LGA from the aorta
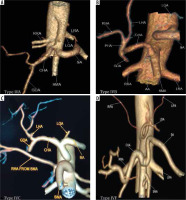
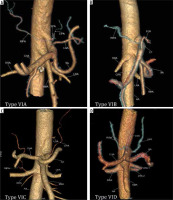
Figure 6
Computed tomography angiography images showing: A) common inferior phrenic artery (CIPA) from celiac artery (CA) (Type VIA), B) right inferior phrenic artery (RIPA) from CA (Type VIB), C) left inferior phrenic artery (LIPA) from CA (Type VIC), and D) bilateral IPA from CA (Type VID)
A study conducted by Juszczak et al. in 1000 patients found normal trifurcation in 93% of cases, unlike in our study where it was seen in 38.85% of cases. We found that there were significant differences in incidences of Type IIc (1.4%), Type IVa (1.1%), and Type IVb (1.7%) variations, as reported by Juszczak et al., compared to our study, except in the case of Type IIb variant for which the difference was negligible. Also, they did not report any case of Type IVf while we found this variant in 0.38% of cases [32].
Dos Santos et al. performed a review study of 12 chosen articles each with its own sample, a method for evaluating anatomical structures, and primary findings. In most investigations, the typical anatomical pattern was the most common (75.0%). In 41.7% of the cases, CT was not present. The existence of CT with bifurcation was the most common anatomical difference (66.7%). The origin of the common and splenic hepatic arteries from the mesenteric arteries was also discovered (25.0%). Other observations included the occurrence of only one branch (16.7%) and quadrifurcation (8.33%) [33].
Clinical importance of CA variations
A comprehensive understanding of the origin, branches, and variations of the CA is of paramount importance while performing abdominal surgeries and for planning digital subtraction angiography (DSA) and interventional procedures like trans-arterial chemoembolization (TACE). In the Appleby procedure, which is a radical gastric surgery involving gastrectomy and splenectomy, Type IIc and III are favourable anatomies because injury to the CHA is avoided. Types IVa, IVd, IVe, IVf, and IVg require caution because injury to mesenteric vessels is common [34]. Type IIb is advantageous for splenic artery embolization procedure and portal flow modulation procedure (splenic artery ligation) during live donor liver transplant. Types IVf, IVg, and IVe, where the splenic artery is from the mesenteric trunk, complicate these procedures [35-37]. In pancreatic surgeries such as Whipple’s procedure, GDA is usually ligated. Caution should be taken with Types IVb and IVf anatomy because there is a high chance of injury to the CHA and liver necrosis if not reconstructed [38].
In liver donor transplants, type IVc is advantageous in right lobe donors. Left hepatic artery (LHA) from CHA, segment 4 from CHA, or segment 2 and 3 from LGA is advantageous in left lobe donors because the risk of injury to the remnant liver is lower. Also, in the case of arterial dissections, these replaced arteries are used as alternatives instead of the complex arterial anastomosis [34]. In diseased donor liver transplants, the graft CA is usually harvested from the arterial reconstruction. Type III, IVc, and IVb variants must be identified because multiple arterial reconstructions are required for good graft outcome [39]. The splenic flexure, a watershed area that receives blood from 2 arteries (SMA and IMA), is more susceptible to damage in cases of ischemic diseases; very rarely, this area may receive blood from the celiomesenteric trunk. Therefore, a study of such variations using CTA is important before performing any procedure. In patients where GI bleeding is difficult to localize, arterial phase CT is helpful because it can also reveal the anomalous origin of arteries [40].
Type VI: IPA is an important extra-hepatic collateral vessel in cases of hepatocellular carcinomas, especially those located in the peripheral segments or bare areas of the liver. CTA is used for precise identification of the artery supplying the tumour and to ensure complete embolization. IPA is considered to be the extrahepatic tumour arterial supply if it measures more than 2.5 mm [21]. Awareness of the LIPA variants is useful in gastrectomy, hiatus hernia repair, and for surgeries around the gastroesophageal junction to prevent inadvertent injury to the LIPA and also to ligate the collaterals from the LIPA supplying these areas, if any [30]. Rarely, in chronic pancreatitis, haemo-ptysis occurs due to pleuro-pancreatic fistula where there is a systemic-pulmonary anastomosis between the LIPA and bronchial artery. In such conditions, haemoptysis is managed by embolization of the IPA, for which awareness of such variants is important [41].
Conclusions
In the present study, we have tried to classify CA branching pattern into 6 main types with their subtypes and proposed an embryological basis for such variations in the CA. We analysed previous studies from the literature, including cadaveric, post-mortem, CTA, and digital subtraction angiography studies. Variations of CA classifications reported to date were analysed, and found that it could not encompass all variants of CA branching patterns. So, we have proposed a new classification that incorporates most of the variants. We reiterate the clinical importance of anatomical variants like CA, IPA, and SMA. Today, in the era of minimally invasive procedures, and interventional and robotic surgeries, it is essential to understand the normal anatomy and variations of the CA. Knowledge of these variations is important for an accurate diagnostic approach, which will help surgeons and interventional radiologists to avoid any iatrogenic complications while performing abdominal and thoracic procedures.