Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / BASIC RESEARCH
Overall survival of patients with EGFR mutation-positive non-small-cell lung cancer treated with erlotinib, gefitinib or afatinib under drug programmes in Poland – real-world data
1
Division of Quality Services, Procedures and Medical Standards, Medical University of Lodz, Lodz, Poland
2
UHE Satelite Campus in Warsaw, University of Humanities and Economics in Lodz, Poland
3
Department of Pharmacoeconomics, Institute of Mother and Child, Warsaw, Poland
4
Analysis and Strategy Department, Central Office, National Health Fund, Warsaw, Poland
5
Chemotherapy Department, Copernicus Memorial Hospital, Medical University of Lodz, Lodz, Poland
6
Comprehensive Cancer Center and Traumatology, Copernicus Memorial Hospital, Medical University of Lodz, Lodz, Poland
Submission date: 2018-06-21
Final revision date: 2018-10-26
Acceptance date: 2018-11-13
Online publication date: 2019-01-22
Publication date: 2021-11-09
Corresponding author
Gabriela Majkut
Analysis and Strategy Department, Central Office, National Health Fund, 2 Hankiewicza St, 02-103 Warsaw, Poland
Analysis and Strategy Department, Central Office, National Health Fund, 2 Hankiewicza St, 02-103 Warsaw, Poland
Arch Med Sci 2021;17(6):1618-1627
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The aim of the study was to estimate the overall survival of patients with EGFR mutation-positive non-small-cell lung cancer treated with erlotinib, gefitinib or afatinib.
Material and methods:
Real-world patients who received afatinib, erlotinib or gefitinib between 1 July 2012 and 30 October 2017 were analysed in five subgroups.
Results:
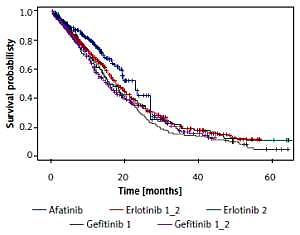
Among 267 patients treated with afatinib financed as the first line of treatment, 76 (28.46%) deaths occurred. Median observation time was 12.8 months (95% CI: 11.2–13.9). Median OS was 22.8 months (95% CI: 19.2–27.1). Among 83 patients who received erlotinib financed exclusively as the second line of treatment the number of deaths was 74 (89.16%). Median observation time was 64.3 months (95% CI: 60.4–64.6). Median OS was 16 months (95% CI: 13.2–22.9). Among 622 patients who received erlotinib financed both as first and second line treatment, there were 400 (64.3%) deaths. Median observation time was 33.3 months (95% CI: 31.2–37.6). Median OS was 17.8 months (95% CI: 16.4–19.7). Among 137 patients who received gefitinib financed only as the first line of treatment, there were 128 (93.4%) deaths. Median observation time was 58.3 months (95% CI: 49.4–62.5). Median OS was 16 months (95% CI: 13.8–19.7). Among 348 patients who received gefitinib financed both as the first and second line of treatment the number of deaths was 208 (59.8%). Median observation time was 23.7 months (95% CI: 20.7–28.7). Median OS was 15.5 months (95% CI: 12.9–17.5).
Conclusions:
Our real-world data regarding OS confirm the benefits found in clinical trials from the use of afatinib, erlotinib or gefitinib. However, the lower overall survival rate of Polish patients compared to similar studies from other research centres suggests the need for deeper investigation of this issue.
The aim of the study was to estimate the overall survival of patients with EGFR mutation-positive non-small-cell lung cancer treated with erlotinib, gefitinib or afatinib.
Material and methods:
Real-world patients who received afatinib, erlotinib or gefitinib between 1 July 2012 and 30 October 2017 were analysed in five subgroups.
Results:
Among 267 patients treated with afatinib financed as the first line of treatment, 76 (28.46%) deaths occurred. Median observation time was 12.8 months (95% CI: 11.2–13.9). Median OS was 22.8 months (95% CI: 19.2–27.1). Among 83 patients who received erlotinib financed exclusively as the second line of treatment the number of deaths was 74 (89.16%). Median observation time was 64.3 months (95% CI: 60.4–64.6). Median OS was 16 months (95% CI: 13.2–22.9). Among 622 patients who received erlotinib financed both as first and second line treatment, there were 400 (64.3%) deaths. Median observation time was 33.3 months (95% CI: 31.2–37.6). Median OS was 17.8 months (95% CI: 16.4–19.7). Among 137 patients who received gefitinib financed only as the first line of treatment, there were 128 (93.4%) deaths. Median observation time was 58.3 months (95% CI: 49.4–62.5). Median OS was 16 months (95% CI: 13.8–19.7). Among 348 patients who received gefitinib financed both as the first and second line of treatment the number of deaths was 208 (59.8%). Median observation time was 23.7 months (95% CI: 20.7–28.7). Median OS was 15.5 months (95% CI: 12.9–17.5).
Conclusions:
Our real-world data regarding OS confirm the benefits found in clinical trials from the use of afatinib, erlotinib or gefitinib. However, the lower overall survival rate of Polish patients compared to similar studies from other research centres suggests the need for deeper investigation of this issue.
REFERENCES (22)
1.
Sharma D, Newman T, Aronow W. (2015). Lung cancer screening: history, current perspectives, and future directions. Arch Med Sci. 11: 1033-43.
2.
Yamada K, Miyamoto S, Azuma K, et al. (2018). A multicenter phase II study of low-dose erlotinib in frail patients with EGFR mutation-positive, non-small cell lung cancer: Thoracic Oncology Research Group (TORG) trial 1425. J Clin Oncol. 36: abstr 9063.
3.
Aren O, Silva R, Cerda H, et al. (2015). Single institution experience with 75 mg dose of erlotinib in Latin American patients with mutated metastatic non-small cell lung cancer. J Clin Oncol. 33: abstr 8075.
4.
Treatment of. (). http://onkologiaonline.pl/upload/obwieszczenie/2017.12.21/b/b.63.pdf. th.
5.
Treatment of. (). http://onkologia-online.pl/upload/obwieszczenie/2017.12.21/b/b.6.pdf. Accessed: March 6th 2018.
6.
Mok TS, Wu YL, Thongprasert S, et al. (2009). Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361: 947-57.
7.
(). https://clinicaltrials.gov/ct2/show/results/NCT00322452?sect=X01256. Accessed: March 6th 2018.
8.
Rosell R, Carcereny E, Gervais R, et al. (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13: 239-46.
9.
Costa C, Molina MA, Drozdowskyj A, et al. (2014). The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. 20: 2001-10.
10.
Zhou C, Wu YL, Chen G, et al. (2011). Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12: 735-42.
11.
Zhou C, Wu YL, Chen G. (2015). Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 26: 1877-83.
12.
Maemondo M, Inoue A, Kobayashi K, et al. (2010). Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 362: 2380-8.
13.
Inoue A, Kobayashi K, Maemondo M, et al. (2013). Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 24: 54-9.
14.
Mitsudomi T, Morita S, Yatabe Y, et al. (2010). Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11: 121-8.
15.
Yoshioka H, Mitsudomi T, Morita S, et al. (). http://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.8117. Available at:.
16.
Wu YL, Zhou C, Liam CK, et al. (2015). First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 26: 1883-9.
17.
Yang JC, Wu YL, Schuler M. (2015). Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 16: 141-51.
18.
Paz-Ares L, Tan EH, O’Byrne K, et al. (2017). Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 28: 270-7.
19.
Fujiwara A, Yoshida M, Fujimoto H. (2018). A Retrospective comparison of the clinical efficacy of gefitinib, erlotinib and afatinib in Japanese patients with non-small cell lung cancer. Oncol Res. 26: 1031-6.
20.
Yang Z, Hackshaw A, Feng Q, et al. (2017). Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 140: 2805-19.
21.
Sluga R, van den, Roepman P, et al. (2018). Utilization of molecular testing and survival outcomes of treatment with first- or second-line tyrosine kinase inhibitors in advanced non-small cell lung cancer in a Dutch population. Anticancer Res. 38: 393-400.
22.
Sebastian M, Schmittel A, Reck M, et al. (2014). First-line treatment of EGFR-mutated non-small cell lung cancer: critical review on study methodology. Eur Respir Rev. 23: 92-105.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.