Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
GASTROENTEROLOGY / CLINICAL RESEARCH
Overall treatment outcome – analysis of long-term results of rectal cancer treatment on the basis of a new parameter
1
Chair of Surgical Oncology, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Poland
2
Department of Surgical Oncology, Oncology Center – Prof. Franciszek Lukaszczyk Memorial Hospital, Bydgoszcz, Poland
3
Department of Clinical Oncology, Oncology Center – Prof. Franciszek Lukaszczyk Memorial Hospital, Bydgoszcz, Poland
4
Department of General, Oncological and Vascular Surgery, 5th Military Clinical Hospital in Kraków, Poland
5
Chair of Surgery, Faculty of Medicine and Health Sciences, Andrzej Frycz Modrzewski Krakow University, Poland
6
National Institute of Oncology, Maria Skłodowska-Curie Memorial, Scientific Editorial Office, Poland
7
Department of Breast Cancer and Reconstruction Surgery, Oncology Center – Prof. Franciszek Lukaszczyk Memorial Hospital, Bydgoszcz, Poland
Submission date: 2017-05-26
Final revision date: 2017-10-16
Acceptance date: 2017-11-12
Online publication date: 2020-04-08
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):825-833
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Outcomes of rectal cancer treatment depend on preoperative staging and the effectiveness of treatments. According to disease staging, different variants of combined therapy (surgery, chemo- and radiotherapy) are used. Available parameters such as overall survival rates and disease- free survival rates as well as the presence of recurrence are inaccurate and should be jointly considered.
Material and methods:
Data from 138 patients with rectal cancer (I–III WHO), who were radically operated on in the period 2001–2004 in Bydgoszcz Oncology Centre were analysed. Among this group 84 patients were radically operated on one week after preoperative radiotherapy 5 × 5 Gy (sRT). We established a new parameter, the overall treatment outcome (OTO), based on the finding that there was no recurrence (local recurrence, distant metastases) of the disease within 5 years, which is generally considered a good result for the treatment of rectal cancer.
Results:
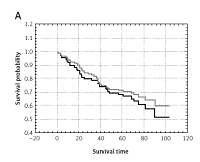
Among all patients (n = 138) and patients following sRT (n = 84) 7.4%...5.9% local recurrence and 24%...29% distant metastases were observed in 5-year follow-up. Recurrence was found in 30% and 31% of patients, respectively. Analysis of results on the basis of the OTO parameter demonstrated that among all groups of patients a worse treatment outcome is related to the number of lymph nodes involved, pN, pT, cancer stage (WHO) and to pN and patient age in the sRT group (p < 0.005).
Conclusions:
In using a combined therapy, it is possible to optimise rectal cancer treatment outcomes. The OTO parameter is a useful tool for defining these results of cancer combination treatment.
Outcomes of rectal cancer treatment depend on preoperative staging and the effectiveness of treatments. According to disease staging, different variants of combined therapy (surgery, chemo- and radiotherapy) are used. Available parameters such as overall survival rates and disease- free survival rates as well as the presence of recurrence are inaccurate and should be jointly considered.
Material and methods:
Data from 138 patients with rectal cancer (I–III WHO), who were radically operated on in the period 2001–2004 in Bydgoszcz Oncology Centre were analysed. Among this group 84 patients were radically operated on one week after preoperative radiotherapy 5 × 5 Gy (sRT). We established a new parameter, the overall treatment outcome (OTO), based on the finding that there was no recurrence (local recurrence, distant metastases) of the disease within 5 years, which is generally considered a good result for the treatment of rectal cancer.
Results:
Among all patients (n = 138) and patients following sRT (n = 84) 7.4%...5.9% local recurrence and 24%...29% distant metastases were observed in 5-year follow-up. Recurrence was found in 30% and 31% of patients, respectively. Analysis of results on the basis of the OTO parameter demonstrated that among all groups of patients a worse treatment outcome is related to the number of lymph nodes involved, pN, pT, cancer stage (WHO) and to pN and patient age in the sRT group (p < 0.005).
Conclusions:
In using a combined therapy, it is possible to optimise rectal cancer treatment outcomes. The OTO parameter is a useful tool for defining these results of cancer combination treatment.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.