Introduction
Colonoscopy is the most commonly performed endoscopic examination worldwide and is considered the gold standard for colorectal cancer (CRC) prevention [1]. CRC screening has been successful in reducing colorectal cancer incidence and mortality by increasing the proportion of lesions diagnosed at an early stage and facilitating removal of pre-neoplastic lesions [2]. The quality of endoscopic examination and treatment is affected by a number of factors that are verified by recognized parameters such as cecal intubation rate (CIR) and cecal intubation time (CIT), withdrawal time (WT), polyp detection rate (PDR) and adenoma detection rate (ADR). Almost a quarter of existing colonic adenomas remain undetected during a screening colonoscopy, while more recent studies raise that percentage even up to 40% [3–6]. In addition to inadequate bowel preparation, inability to reach the cecum (incomplete colonoscopy), quick WTs (< 6 min) and patient-related factors, the primary factor accountable for missed colorectal adenomas and early cancers is the relatively narrow field of view (140–170°) of standard forward viewing (SFV) colonoscopes [7]. Anatomical sites such as the proximal aspect of colonic folds, anatomical flexures and the area around the ileocecal valve tend to be hidden from SFV colonoscopes. In an effort to eliminate these limitations, novel endoscopes with a wider field of view have been manufactured, allowing meticulous inspection of the proximal aspect of haustral folds [8]. These include the Fuse Full Spectrum Endoscopy colonoscopy platform (EndoChoice Inc., Alpharetta, GA, USA).
Aim
The aim of this study was to assess the suitability of panoramic view colonoscopy (PVC) for the detection of colorectal lesions and to analyze the functionality of this special endoscope series regarding CIR, CIT and WT as compared to standard forward viewing colonoscopy (SFV).
Material and methods
We explored the efficacy of the PVC in a randomized single-center feasibility study performed between July 1 and December 20, 2017. The study was conducted at a private independent hospital that performs more than 11 000 colonoscopies per year. The study was approved by the local ethics committee and was conducted in accordance with the principles of the Declaration of Helsinki. As a clinical trial, the study was registered in a centralized clinical trials registry (ClinicalTrials.gov – NCT02929381).
The study enrolled 421 patients aged 18–80 years who were eligible for colonoscopic examination performed for colorectal cancer screening, polyp surveillance or diagnostic evaluation. Patients with prior abdominal surgery, colorectal resections or inflammatory bowel disease were excluded from the study. Patients were randomized to either SFV colonoscopy (Olympus Evis Exera III, CF-HQ190L) or the novel wide-angle PVC (FUSE colonoscope CDVL slim c38).
All patients were given the same bowel preparation guidelines based on oral ingestion of 420 g of polyethylene glycol (PEG) in 4 l of water taken in 4 doses every 6 h 1 day before the examination. The large bowel was prepared according to the split-dose principle and colonoscopy was performed in the afternoon. Each colonoscopy was performed by one of three experienced endoscopists (each of whom had performed more than 5000 colonoscopies). A standard, commercially available high-definition colonoscope (Olympus 190 series Exera III CF-HQ190L with NBI system and DF capability) was used for all SFV colonoscopies in this study. This endoscope utilizes an advanced Dual Focus optical system and Narrow Band Imaging (NBI) to produce clear images for observation. It has a single camera at the tip of the scope, which allows a 170° wide view. The working length of the scope is 168 cm. The working channel width is 3.7 mm, and the outer diameter of the scope is 13.2 mm.
In patients randomized to the PVC group, the Fuse Full Spectrum Endoscopy colonoscopy platform (EndoChoice Inc., Alpharetta, GA, USA) was used. This platform comprises a video colonoscope and a processor [9–11]. The Fuse colonoscope is a standard (168 cm working length, 12.8 mm scope outer diameter) flexible, reusable, reprocessable colonoscope intended for diagnostic visualization and therapeutic interventions. The Fuse colonoscope provides a high resolution, 330° field of view while maintaining all standard colonoscope capabilities and maneuverability including full-tip flexion, 3.8 mm working channel, air or CO2 insufflation and water jet irrigation. Thus, the Fuse colonoscope maintains identical technical features when compared with the current industry standard forward viewing colonoscopes. Full spectrum endoscopy is achieved by the use of three imagers and LED groups positioned at the front and on both sides of the colonoscope tip. Endoscopic images are displayed on three contiguous video monitors. The left, center and right video monitors correspond with the colonic images transmitted from the left facing, forward facing, and right facing lenses, respectively (Photo 1).
After cecal intubation, the colonic mucosa was carefully visualized using white light while withdrawing the colonoscope. All polyps detected during the procedure were documented for size, location, and morphology according to the Paris classification. Polyps with a diameter of < 3 mm were resected using biopsy forceps without diathermy; polyps measuring 4–7 mm were resected by cold snare polypectomy; larger lesions were removed by endoscopic hot snare polypectomy or endoscopic mucosal resection. Resected specimens were stained with hematoxylin and eosin and reviewed by two expert gastrointestinal pathologists with more than 10 years of gastrointestinal pathology experience. Histopathologists were blinded to the type of endoscope used for the study. All polyps removed were classified as hyperplastic polyps, adenomas or advanced adenomas based on histopathological examination. All images were captured and stored as high-definition JPEG files (200–300 kb, 1280 × 1024 pixel array, 32-bit RGB representation).
Statistical analysis
The materials acquired in this study were systematized and analyzed, and the distribution of the variables was established. Because the analyzed parameters do not have a normal distribution, nonparametric tests were used in the analysis. Qualitative variables were compared using the independent χ2 test. The Mann-Whitney U test was used to compare quantitative variables between two groups. The Kruskal-Wallis test was used for comparisons of quantitative data in more than two groups. The significance threshold was established at p < 0.05.
Results
There were 214 patients examined with SFV and 207 with PVC. The two groups were comparable in gender (p = 0.559) and BMI (min. 17 kg/m2, max. 41 kg/m2, p = 0.190) (Table I). There were 128 females and 86 males in the SFV group and 118 females and 89 males in the PVC group. The mean age of patients was 64 ±12.26 years. Bowel preparation in both groups was similar, with a mean score above 8 in both groups, the median being 8. The mean cecal intubation time was significantly longer using the PVC endoscope as compared to the SFV endoscope: it measured 234 s with SFV vs. 311 s with PVC (p < 0.001). Minimum CIT in the SFV group was 60 s, whereas in the PVC group the min CIT was 90 s. Maximum CIT in the SFV was 520 s, and in the PVC group 585 s (Table II). There were no significant differences in WT between the two groups (mean WT 509.66 s in SFV vs. 510.23 s in PVC group, p = 0.997). Minimum WT in both groups was 360 s, and maximum WT was 970 in both groups (Table III). The main end-points of this study were polyp and adenoma detection rates. Taking into consideration the whole colon, the polyp detection rate was higher in the PVC group (40.1%) compared to the SFV (34.8%) group, although statistical difference was not achieved (p = 0.242). The adenoma detection rate was similar in both groups (26.17% in SFV vs. 27.05% in PVC, p = 0.837). The advanced adenoma detection rate (aADR) was 14.02% in the SFV group and 14.01% in the PVC group (p = 0.998) (Table IV). No difference was found in terms of diverticuli detection rate (43% in SFV vs. 45% in PVC group, p = 0.617) in the whole colon.
Table I
Group characteristics
| Group | Sex | N | Age | BBPS | BMI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Min. | Max. | Mean | SD | ||||
| SFV | F | 128 | 214 | 62.34 | 11.46 | 8.08 | 1.13 | 17 | 41 | 27.34 | 5.84 |
| M | 86 | 62.71 | 11.94 | 8.03 | 1.16 | 17 | 41 | 28.27 | 5.33 | ||
| PVC | F | 118 | 207 | 65.27 | 12.90 | 8.17 | 1.20 | 17 | 40 | 27.25 | 4.29 |
| M | 89 | 66.03 | 12.54 | 8.04 | 1.22 | 18 | 38 | 26.54 | 4.23 | ||
Table II
Cecal intubation time
| Group | Sex | Min. CIT [s] | Max. CIT [s] | Mean CIT [s] | SD | ||||
|---|---|---|---|---|---|---|---|---|---|
| SFV | F | 60 | 60 | 520 | 520 | 236.68 | 234.35 | 97.75 | 96.75 |
| M | 60 | 510 | 230.87 | 95.71 | |||||
| PVC | F | 90 | 90 | 585 | 585 | 297.92 | 310.94 | 119.77 | 120.48 |
| M | 100 | 560 | 329.6 | 119.91 | |||||
| p < 0.001 | |||||||||
Table III
Withdrawal time
| Group | Sex | Min. WT [s] | Max. WT [s] | Mean WT [s] | SD | ||||
|---|---|---|---|---|---|---|---|---|---|
| SFV | F | 360 | 360 | 970 | 970 | 508.03 | 509.66 | 113.82 | 117.53 |
| M | 360 | 915 | 512.09 | 123.48 | |||||
| PVC | F | 365 | 360 | 870 | 970 | 495.33 | 510.23 | 102.78 | 118.89 |
| M | 360 | 970 | 529.99 | 135.45 | |||||
| p = 0.997 | |||||||||
Table IV
Polyp, adenoma, advanced adenoma and diverticuli detection rates
Segments of the colon
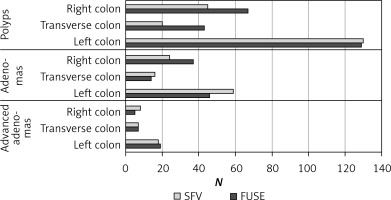
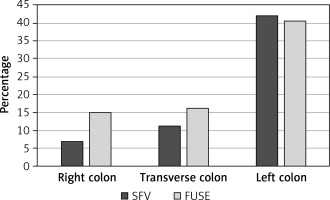
A detailed analysis of the incidence of polyps, adenomas and diverticuli in particular segments of the colon (right, transverse, left) was performed (Figures 1 and 2). Incidence of adenomas in the right part of the colon (p = 0.832), the transverse part (p = 0.904) and the left part (p = 0.431) was similar in both groups. Incidence of advanced adenomas was also comparable between the groups in the respective parts of the colon (right side p = 0.732, transverse p = 0.631 and left p = 0.681). Significantly more polyps were found during PVC endoscopy in the transverse colon compared to the SFV colonoscopy (43 vs. 20, p = 0.006). No difference in the number of detected polyps was found in the left and right side of the colon (p = 0.059 and p = 0.858, respectively). Additionally, we observed that PVC allowed for detection of significantly more diverticuli in the right side of the colon in comparison to SFV endoscopy (p = 0.009). In the remaining parts of the colon, the number of the diverticuli discovered was similar (p = 0.121 in the transverse and p = 0.759 in the left part).
Discussion
PVC was introduced as a new advanced imaging method that could potentially improve the adenoma detection rate and reduce the adenoma miss rate. Multiple studies in varied patient populations have shown significant adenoma miss rates during forward viewing, optical colonoscopy [11–15]. Missed adenomas are one of the biggest limitations to the colonoscopy examination and can lead to interval colorectal cancers [16, 17]. Since the introduction of PVC, many studies have proven its efficacy in decreasing the adenoma miss rate; nevertheless, its production was stopped 3 years after the market premiere. Standard colonoscopy often requires multiple manipulations of the colonoscope by the examiner, including efforts to flatten folds and straighten flexures, as well as prolonged retroflexion of the colonoscope itself. All these maneuvers are performed to reveal polyps hidden in the ‘blind spots’ and increase ADR, but they are technically demanding, time-consuming and pose some risk to the patient. The great advantage of PVC is that it allows one to see the lesions located in these blind spots by using the side cameras.
In 2014 Gralnek et al. conducted an international, multicenter, randomized back-to-back study to investigate whether PVC detects more adenomas in comparison to SFV colonoscopy [18]. In this study, the PVC system had a significantly lower miss rate compared to SFV endoscopy for adenomas (7% vs. 41%, p < 0.0001) and polyps (10% vs. 43%, p < 0.0001). However, in our study the PVC system allowed for detection of more polyps and diverticula in certain parts of the colon without affecting ADR and aADR.
Gralnek et al. previously reported a 100% success rate in cecal intubation using PVC during a prospective, single-center pilot study [19]. While the CIR was comparable to that of SFV in our study, the CIT with PVC was significantly longer than with SFV, which may stem from the fact that at first it may be somewhat confusing to follow all three screens with live images from different cameras at the tip of the scope, and there is a learning curve to it. Regardless, this novel platform has been proven to be safe and feasible with CIR at almost 100% in two nonrandomized studies, which seems to be consistent with our results [19, 20].
In an interesting meta-analysis by Facciorusso et al. all randomized studies comparing PVC with SFV endoscopy published until May 2019 were taken into account. Primary outcomes in the study were polyp detection rate and adenoma detection rate, and secondary outcomes were cecal intubation time and total colonoscopy time. It was found that even though the adenoma and polyp detection rates were not significantly different between the groups, the PVC group had a significantly lower adenoma miss rate (risk ratio 0.35, 0.25–0.48, p < 0.01). What is also interesting in this meta-analysis is that it shows that there was no difference in cecal intubation time between the groups and, most interestingly, the total colonoscopy time was significantly shorter in the PVC group (mean difference: –2.60, 95% CI: –4.60, –0.61; p = 0.01) [21]. This is somewhat contradictory to many single studies, including our study, which showed prolonged CIT and total colonoscopy time in the PVC group. This might however stem from the fact that the technology at first is somewhat hard to tame and there is an ‘adaptation and tolerance curve’ to it, rather than a genuine learning curve – even very experienced endoscopists find it quite confusing and tiresome to use PVC endoscopy at first. This meta-analysis however has shown that after mastering the technique, PVC can not only decrease the adenoma miss rate, but can also decrease the cecal intubation time and total colonoscopy time, which is an important factor taken into consideration in all high volume endoscopy centers.
On the other hand, there are also studies that fail to prove the benefit of this technology. Nunez-Rodriguez et al. reported no benefit of SVC over SFV colonoscopy regarding polyp detection rate and adenoma detection rate in the whole colon, as well as in a detailed analysis by lesion size or colon section. They did however report a significantly prolonged cecal intubation time in the PVC group (PVC 6.2 min vs. standard colonoscopy 4.2 min; p < 0.001) [22]. It is consistent with our study regarding intubation time, but we observed a significantly higher polyp detection rate in the transverse colon and a higher diverticuli detection rate in the right part of the colon using PVC in comparison to SFV endoscopy.
Another interesting factor that was assessed in some studies is the experience of the physician performing the examination. Following three life screens at the same time and merging those images into one piece of information may be tricky for the examiner, who is used to looking at one live screen showing a picture from a single camera at the tip of the scope – this seems more natural for everybody, and switching to PVC with its three live screens poses a lot of discomfort for the examiner at first. A study by Ito et al. showed that the preliminary evaluation of the PVC system by the examiners was not very satisfactory. It was rated higher than SFV in only three categories: field of view, screening and polyp surveillance. In the remaining 11 categories it was rated as inferior to SFV colonoscopy [23].
In assessing articles about PVC and its influence on ADR and PDR one has to remember that most comparative studies are performed in a tandem-examination fashion – one colonoscopy using one technology is followed by another colonoscopy with a different technology. Such tandem colonoscopy always poses bias in the results, as it has been proven that, using any technology, more lesions are found in the second examination in the same patient.
This panoramic view endoscopy platform may have a role not only in detecting lesions hidden behind the fold of the intestine. One has to remember that lesions that have been recognized as a significant precursor of advanced colorectal cancer are so-called laterally spreading tumors (LSTs), which tend to be overlooked in colonoscopy because of the unique morphological features [24]. A wider viewing angle and dedicated side cameras may potentially increase the chance of detecting LSTs, which are a significant risk for the patient and can be overlooked due to their non-polypous growth pattern.
The field of endoscopy is evolving rapidly, with more and more lesions being qualified for endoscopic removal rather than a more invasive surgical procedure. The long-term results of both endoscopic mucosal resection and endoscopic submucosal dissection have proven them to be reliable techniques with a low rate of complications and short hospital stay in the treatment of colon and rectal lesions, including early stage carcinomas [25]. Innovation in this field is inevitable, and PVC is one of its faces.
Our general impression of the scope insertion, the operation of angles, release of angles and retroflection in the rectum was rated lower than that of SFV. We noted a higher stiffness of the FUSE shaft, lower radius at the full flexure and its overall poorer flexibility, which led to more difficulties during the examination and higher CIT. However, we realize that the colonoscopists’ lack of experience with the PVC system may have contributed to the worse user experience compared to that of SFV.
Moreover, we found that the removal of lesions identified on the side monitor was slightly troublesome. This study proves that the controversies around this device are justified, as the results of its contribution to a higher adenoma detection rate and therefore a possibility to decrease long-term colorectal cancer mortality are contradictory and remain uncertain. However, we believe that the decision to stop production of the FUSE devices is somewhat premature, as there are many studies showing its positive impact on efficacy of colonoscopy screening.
Obviously this study has limitations. One such limitation is the learning curve of the use of PVC – the examiners that participated in the study were all highly experienced, with at least 5000 examinations performed prior to the study. One has to keep in mind that all those prior examinations were SFV colonoscopies, and the ‘practice’ period with the PVC was no more than 10 examinations per physician – which might have affected the results of the study, because it definitely takes some time to get used to the new platform. Another limitation, quite controversially, might be the randomized character of the study – it has been widely accepted to perform colonoscopy platforms in a ‘tandem-examination’ manner, with each platform being used in the same patient back-to-back. The idea behind this is to reduce the bias of patient-related conditions that influence the quality of the examination. Other than that, we believe that the study was robust and reliable in its structure.