Introduction
The hemostatic balance in the human body is the result of a precisely maintained equilibrium between the processes of fibrin formation and fibrinolysis [1]. Delayed blood flow, changes in vessel wall or blood composition can disturb this balance and lead to thrombotic complications. Despite their seemingly identical manifestation, namely the formation of occlusive thrombi, the pathophysiological mechanisms that lead to these complications may be fundamentally different [2]. For example, the main cause of venous thromboembolism is delayed blood flow in the peripheral veins. The initiating moment in arterial thrombosis is platelet adhesion and aggregation due to endothelial damage. These fundamental differences are of major clinical importance because they predetermine a fundamentally different approach to antithrombotic therapy. Awareness of hemostasis in various prothrombotic conditions is the key to creating effective antiplatelet or anticoagulant therapies and, respectively, to successful prevention and treatment of thromboembolic events.
As a common pathophysiological mechanism of myocardial infarction, ischemic stroke, deep vein thrombosis, and pulmonary embolism, thrombosis is a leading cause of death [3]. As a well-defined prothrombotic condition, atrial fibrillation remains a major risk factor for two of these diseases: ischemic stroke and peripheral arterial thromboembolism. A systematic analysis of the Global Burden of Disease Study 2010 showed that the mortality associated with AF embologenic risk has increased two-fold in the last two years, reaching an age-adjusted mortality rate of 1.7 per 100,000 [4]. It is believed that one-quarter to two-thirds of the AF population are patients with transient or paroxysmal atrial fibrillation (PAF) [5]. This raises significant interest in PAF embolic risk and associated hemostatic changes.
Generally, PAF thromboembolic incidents with onset < 48 h have a low incidence rate (0–0.9%); therefore acute cardioversion is justified for the general population [6–9]. However, the risk appears to vary substantially depending on the patient’s characteristics and the onset of the arrhythmia even within those first 48 h of the disease [10]. It is unacceptably high in patients with heart failure and diabetes mellitus (up to 9.8%) compared to patients < 60 years of age without heart failure. After the 12th h from the onset of arrhythmia, the risk reaches 1.1% compared to 0.3% in the first few hours of the disease. Very little is clear about the hemostatic changes in the early hours of AF and their relationship with the onset of the disease.
Coagulation factor VIII (FVIII) and von Willebrand factor (vWF) are important molecules for primary and secondary hemostasis. It is well known that congenital and acquired diseases associated with their deficiency, lack or reduced activity, are associated with significant, sometimes life-threatening bleeding [11]. They play an important role in the thrombus formation process and their hemostatic functions are closely related and interdependent. FVIII is a key factor in the coagulation cascade and therefore its activity and concentration are determinants of hemostatic balance [12]. Most frequently activated by thrombin, FVIIIa has the function of a non-enzymatic FIX co-factor, causing significant activation and a burst of thrombin formation, the molecule that directly stimulates fibrin synthesis. The von Willebrand factor is directly responsible for the FVIII procoagulant function. By binding it into the non-covalent FVIII/vWF complex, it stabilizes it and significantly prolongs its circulation half-life. vWF plasma levels are associated with the presence of endothelial damage, since endothelial cells are their primary source [13].
The pivotal role of FVIII and vWF in hemostasis and the lack of sufficient knowledge of it in the early hours of PAF were prerequisites for our study.
The aim of the study was to study vWF and FVIII plasma levels and activity in the first hours (up to 24 h) of the clinical manifestation of PAF.
Material and methods
Study poulation
Patients over 18 years of age with PAF episode onset of < 24 h prior to hospitalization were sequentially screened. They were diagnosed based on electrocardiographic study performed at the hospital. The study was conducted in the Intensive Cardiology Unit of the First Cardiology Clinic at the University Hospital St. Marina, Varna, for the period 10.2010-05.2012 after approval by the local ethics committee (9/14.10.2010) and in accordance with the Declaration of Helsinki [14]. Based on the inclusion and exclusion criteria presented below, 51 patients (26 men and 25 women) with a mean age of 59.84 ±1.60 years (31–77 years) were sequentially selected.
A control group of volunteers with no anamnestic or electrocardiographic AF data was formed, applying the same exclusion criteria (see below). Of the 169 screened volunteers, 52 were selected for the study. Their mean age was 59.50 ±1.46 years (30–76 years), 26 men and 26 women.
The selection of participants was aimed at balancing as much as possible between the two groups the factors influencing the studied indicators and thus eliminating their effect on the results obtained. The two groups were created to be identical in terms of sex, age, body mass index (BMI), deleterious habits, comorbidities and treatment. These are indicators which may affect the values of the studied indicators.
Each participant was enrolled in the study after signing informed consent.
Exclusion criteria
Cardiovascular diseases: ischemic heart disease, heart failure, high-grade and /or uncontrolled hypertension, moderate or severe acquired valve defects, cardiomyopathy, implanted device for the treatment of rhythm-conduction disorders, inflammatory heart disease, congenital heart diseases.
Other diseases – kidney or liver failure, inflammatory and/or infectious diseases, neoplastic and autoimmune diseases, chronic pulmonary insufficiency, endocrine disorders (except for non-insulin dependent, well-controlled DM type 2); previous thromboembolic incidents, bleeding diathesis, miscarriages (for women).
Intake of hormone replacement therapy, contraceptives, oral anticoagulants or antiplatelet drugs, pregnancy, systemic intake of analgesics (incl. NSAIDs), obesity with BMI > 35 kg/m2.
Unsuccessful restoration of sinus rhythm with drugs (propafenone) (for the patient group).
Blood sampling and laboratory measurements
Peripheral venous blood was collected once from each participant in the study in 3.2% sodium citrate Vacutainers (VACUETTE, Greiner Bio-One North America, Inc.). In patients the blood sample was taken immediately after hospitalization and in controls during the outpatient examination. After centrifugation of the samples at 2500 g for 15 min, the plasma was pipetted and stored at –20°C for a maximum of 1 month.
Each indicator was determined twice and the average was taken into account when calculating the results.
Quantification of FVIII was made using an enzyme-linked immunosorbent assay (ASSERACHROM VIII: Ag, Diagnostica Stago, Asnières sur Seine, France). Factor activity was determined photometrically (TECHNOCHROM FVIII, Technoclone, Vienna, Austria). Levels of vWF antigen (vWF : Ag) were examined by TECHNOZYM vWF: Ag ELISA, Technoclone, Vienna, Austria. Activity of the indicator was measured on the basis of its capacity to bind collagen, a major physiological function of vWF that determines its adhesive properties. For this purpose we used an ELISA test (TECHNOZYM vWF : CBA, ELISA, Vienna, Austria).
The intra-assay coefficient of variation (CV) was < 5%, except for vWF : Ag (5.5%). The inter-assay CV was <5%, except for vWF : Ag (5.9%). The lower limit of detection was 0.5% for FVIII : Ag, 0% for FVIII activity, 0.01% for vWF : Ag and 0.01% for vWF : act.
Statistical analysis
All analyses were conducted using Statistica 13.3.0, StatSoft Inc, USA.
Continuous variables were expressed as mean ± standard error of the mean (SE) and categorical variables were expressed as percentage of the total group. Normality of distribution was assessed by the Kolmogorov-Smirnov (Lilliefors) test. Two-tailed Student’s t-test for independent samples was used to compare quantitative variables. Fisher’s exact or Pearson’s χ2 test was used to compare categorical variables and occurrence frequency. Values of p < 0.05 were considered statistically significant. Linear regression analysis was used to identify dependencies in PAF between FVIII and vWF plasma levels and activity (dependent continuous variables) and the explanatory continuous variables age, BMI and onset of the arrhythmia (measured as time spent in AF until hospitalization). The relationship between hemostatic indicators and the categorical independent variables sex and CHA2DS2-VASc score was examined using analysis of variance (ANOVA). Variables showing a level of association p < 0.05 were considered as prognostic.
Results
Study population
There was no statistically significant difference between the patient and control groups in terms of sex, age, comorbidities and treatment, deleterious habits, BMI and main laboratory markers (p > 0.05) (Table I) [15]. The transthoracic echocardiography indicators also showed no significant differences (p > 0.05) (Table II).
Table I
Clinical characteristics of participants
[i] PAF – paroxysmal atrial fibrillation, ACE – angiotensin converting enzyme, CHA2DS2-VASc score – congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease (prior myocardial infarction, peripheral vascular disease, or aortic atherosclerosis), age (65–74 years), and sex category (female). According to CHA2DS2-VASc score, patients were divided into low-risk (score < 2) and high-risk (score ≥ 2) for the emergence of embologenic risk according to the recommendations of the European Society of Cardiology [15].
Table II
Echocardiographic parameters of participants
PAF-episode onset analysis showed that patients were hospitalized between the 2nd and the 24th h from the onset of the arrhythmia, most often at the 5th h. The mean duration of episodes until hospitalization was 8.14 ±0.76 h.
Plasma levels and activity of vWF and FVIII
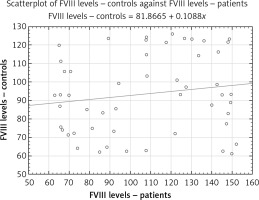
Higher plasma levels of FVIII (107.52 ±3.48% vs. 93.85 ±2.93%, p < 0.05; Figure 1) and higher activity (200.03 ±11.11% vs. 109.73 ±4.90%, p < 0.001; Figure 2) were detected in PAF compared to controls in sinus rhythm. The calculated level of the power was 0.80 (often considered the minimum acceptable level) and 0.99 for FVIII levels and activity, respectively.
Figure 2
Scatterplot of FVIII coagulation activity in PAF patients and controls (FVIII act – FVIII activity)
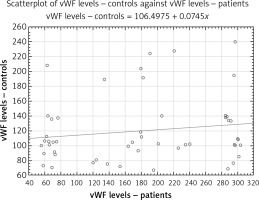
vWF levels were significantly higher in the patient group compared to the control group (178.40 ±12.95% vs. 119.53 ±6.12%, p < 0.001; Figure 3). vWF : CBA was also higher in the PAF group (200.92 ±12.45% vs. 110.80 ±5.14%, p < 0.001; Figure 4). The levels of the power of tests were 0.89 and 0.99, respectively.
Figure 4
Scatterplot of vWF coagulation activity in PAF patients and controls (vWF act – vWF activity measured as collagen-binding activity)
Each scatterplot presents a “round cloud” of points, not a systemic shape (e.g., a straight line, a curve etc.), the variables not being related.
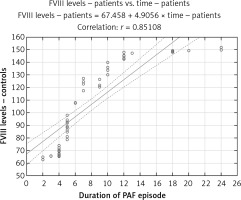
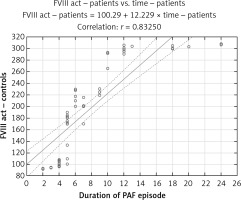
Linear regression analysis showed that patients’ age and BMI were not predictive variables for plasma FVIII levels (r = 0.07, p > 0.05; r = 0.06, p > 0.05, respectively), plasma FVIII activity (r = 0.04, p > 0.05; r = 0.09, p > 0.05, respectively), plasma vWF levels (r = 0.13, p > 0.05; r = 0.07, p > 0.05, respectively), and vWF plasma activity (r = 0.03, p > 0.05; r = 0.02, p > 0.05, respectively). The time from the onset of the arrhythmia was a significant predictor of increased FVIII and vWF plasma levels and activity, and increased PAF duration was followed by increased values of the factors (r = 0.85, p < 0.001, Figure 5; r = 0.83, p < 0.001; Figure 6). No linear relationship was found between vWF levels and activity and time from the arrhythmia onset (r = 0.14, p > 0.05; r = 0.12, p > 0.05).
ANOVA showed that there were no significant differences between male and female FVIII plasma levels and activity in the PAF group (107.72 ±6.92% vs. 107.23 ±5.50%, p > 0.05; 197.28 ±18.19% vs. 202.70 ±13.35%, p > 0.05; respectively), as well as vWF plasma levels and activity (169.07 ±19.43% vs. 187.30 ±17.41%, p > 0.05; 195.55 ±17.25% vs. 206.11 ±18.30, p > 0.05; respectively). No statistically significant difference was found between patients with low (CHA2DS2-VASc score < 2) and high embologenic risk (CHA2DS2-VASc score ≥ 2) in terms of FVIII plasma levels and activity (106.64 ±5.70% vs. 108.27 ±6.67%, p > 0.05; 202.02 ±13.88% vs. 198.14 ±17.50%, p > 0.05; respectively) and vWF plasma levels and activity (191.79 ±17.51% vs. 165.45 ±19.02%, p > 0.05; 202.02 ±18.56% vs. 199.89 ±17.14%, p > 0.05; respectively).
Discussion
Factor VIII is a coagulation factor whose procoagulant effect is well recognized. Its role in prothrombotic syndromes, such as myocardial infarction, deep vein thrombosis (DVT) and acute ischemic stroke, has been demonstrated [16]. Although it circulates in the blood in its inactive “sleeping” form bound in the FVIII/vWF complex, its plasma concentrations directly correlate with the manifestation of a number of thrombotic events. A strong relationship was found between elevated FVIII levels and the incidence of “de novo” or recurrent non-malignant DVT [17]. Every 10 IU/dl above normal limits increased the risk of the disease by 8%. High plasma FVIII activity is commonly observed in patients with cerebral venous thrombosis and is considered a risk factor for this disease [18]. FVIII is associated not only with venous but also with arterial thrombotic complications. It defines an independently significant risk of acute coronary thrombosis in the absence of underlying coronary disease [19]. It is well known that its plasma levels are also associated with the risk of acute ischemic stroke and its consequences [20].
A prerequisite for the scientific interest in the FVIII molecule in the clinical manifestation of AF is the high risk of stroke and thromboembolism that the disease determines. It is well known that FVIII significantly activates the coagulation cascade in response to external and internal procoagulant stimuli [21]. Both thrombin and FXa within the outer TF-FVIIa-FXa initiation complex activate FVIII, and this largely depends on the concentration of the factor itself [22]. In this sense, the functional adequacy of FVIII depends not only on the activity but also on the levels of the molecule. Studies on the coagulation cascade in AF show significantly elevated levels of FVIII [23, 24]. Some of them also establish thromboembolic complications in the presence of high FVIII [25]. The studied populations were heterogeneous in terms of arrhythmia onset (patients with paroxysmal and persistent atrial fibrillation, as well as with restored sinus rhythm) and accompanying diseases. There are no studies on FVIII in PAF. There are no studies in the entire AF population that simultaneously evaluate procoagulant activity and coagulation factor levels. Although there is usually a direct cause-and-effect relationship between them, each of these indicators has its own value and provides specific clinical information [26].
This was the reason to simultaneously examine them in our study. The results showed that there were significant deviations in FVIII during the first 24 h of PAF (Figures 1 and 2). The coagulation activity of the factor was higher compared to the control group (Figure 2). Its plasma levels were also elevated (Figure 1). The unidirectional changes in both indicators and the known causal relationship between them give reason to believe that increased synthesis and secretion of FVIII were observed in PAF under conditions of procoagulant stimulation.
Although not directly involved in the enzyme reactions, but acting as a non-enzymatic co-factor, FVIII is a key coagulation cascade molecule. It is the main molecule responsible for the process of platelet propagation, regardless of the pathway in which the coagulation cascade is activated [16]. In this sense, the observed higher FVIII activity in the patient group implies significant activation of the coagulation cascade and a tendency for hypercoagulability in the first 24 h of the disease. The results of the regression analysis are extremely interesting. They clearly indicate that the clinical manifestation of PAF is associated with increased FVIII activity and levels regardless of age, sex, BMI, and embologenic risk, as determined by the CHA2DS2-VASc score (p > 0.05). PAF itself determines hypercoagulability, increasing over time (Figures 5, 6). Specific to FVIII structure is the volatility of its free form, which greatly limits and reduces its effective participation in the coagulation process. The binding of FVIII to vWF at a 1 : 1 ratio in the non-covalent FVIII/vWF complex prevents early proteolytic degradation of the coagulation factor and prolongs its circulation half-life. The vWF molecule is essential for the functionality of FVIII [27]. This was the reason for the simultaneous study of both vWF and FVIII in our study. High plasma vWF levels were detected (Figure 3). They are consistent and contribute to the unidirectional deviations in FVIII levels. It is reasonable to assume that high FVIII concentration is associated with prolonged plasma half-life and stabilization of the molecule.
vWF is released in the plasma mainly by endothelial cells and, to a lesser extent, by activated platelets in endothelial damage [28]. Due to GpIb conformational changes upon contact with subendental collagen, vWF is activated and has the ability to adhere to platelets. Increased vWF levels in the study patient group are indicative of endothelial damage in the first 24 h of PAF (Figure 3), and its increased activity (Figure 4) of enhanced platelet adhesion.
Changes in vWF, as well as in the aforementioned FVIII, are independent of sex, age, BMI and embologenic CHA2DS2-VASc score (p > 0.05). There was no correlation with the duration of arrhythmia (p > 0.05).
These results suggest a link between these factors and local endothelial burden, observed in high frequency uncoordinated atrial contraction and resulting from AF itself [29]. Changes in vWF are part of the procoagulant state, formed in the early hours of PAF, which is independent of the well-known predictors of sex, age, BMI and CHA2DS2-VASc score.
The aim of our study was to investigate FVIII and vWF plasma activity and levels in the early hours of the clinical manifestation of PAF, namely the first 24 h from the onset of the disease. Therefore, we studied only patients who clearly specified that the arrhythmia started < 24 h prior to hospitalization. In this sense the presented results provide information exclusively about the coagulation in the early hours of clinical manifestation of PAF. They cannot in any way show or predict the coagulation activity in the later hours of the disease. We also believe that these limitations are a ground for new studies, which could examine the dynamics of the system.
In conclusion, the results presented an activated coagulation cascade and endothelial injury, suggesting hypercoagulability still in the early hours of PAF. These changes in PAF did not correlate with CHA2DS2-VASc score risk factors, indicating PAF as a possible independent embolic risk factor.