Introduction
Tissue sampling is usually sought as part of the clinical assessment prior to detection of suspicious pulmonary lesions or mass through any imaging modality. Bronchoscopy is often the first step because it allows not only direct observation of the lung mass to evaluate its characteristics and extent but also collection of tissue samples. Although diagnostic rates of up to 80% have been reported, depending on the method used to acquire tissues, a bronchoscopic biopsy is limited to centrally located tumours that are visible from within the airways [1].
A percutaneous transthoracic needle biopsy (PTNB) is an essential intervention in tissue diagnosis for several pulmonary lesions or nodules that cannot be approached through bronchoscopy. Various imaging techniques have been used as guidance while performing PTNB. Conventional computed tomography (CCT) has been used for the last two decades to guide tissue biopsy for pulmonary nodules. CCT has many advantages, including planning a trajectory that minimises passage through aerated lungs, avoids bullae, fissures, and vessels and allows access to central and small lesions. However, the duration of procedure and non-real-time visualisation of lesions are the major disadvantages of CCT guidance [2].
In the past decade, C-arm cone-beam CT (CBCT) systems, which consist of a C-arm gantry, an X-ray tube, and a flat-panel detector, have been introduced in the radiological intervention field [3-5]. This system offers great flexibility in orienting the detector around the patient to provide 3D reconstructed CT images with real-time fluoroscopy capability. Furthermore, CBCT-guided biopsy offers great accessibility by means of a flexible approach through an open gantry. These advantages of CBCT guidance are expected to improve the diagnostic accuracy and efficacy of PTNB, and operator confidence during the procedure [6-9]. Several studies have reported the diagnostic accuracy and safety of CBCT guidance for biopsies of pulmonary lesions [6-12].
Based on our experience and literature review, only one publication directly compared the diagnostic accuracy and safety between CBCT and CCT guidance for PTNB [13]. However, the study only involved a small number of enrolled patients for each group. The present research aimed to directly compare safety in terms of complication and radiation exposure of patients and diagnostic performance between CBCT and CCT guidance for PTNB of pulmonary nodules.
Material and methods
Patients
This retrospective study involved patients who underwent PTNB from January 1, 2013 to June 30, 2018 in Songklanagarind Hospital, which is a university hospital in southern Thailand. The inclusion criteria included the presence of pulmonary nodules that were not identified by ultrasound and a biopsy procedure by either CCT or CBCT guidance in our hospital from January 1, 2013 to June 30, 2018. The exclusion criterion was lack of histological data. A total of 366 patients with 366 nodules met the inclusion criteria. This study was approved by the Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University (IRB No. 61-060-7-3).
Machine-guided biopsy and general details of the biopsy procedure
From January 1, 2013 to March 31, 2017, PTNB procedures were performed using CCT guidance (64-multidetector CT scanner, Brilliance TMCT, Philips Healthcare, Best, the Netherlands). After March 31, 2017, our unit acquired new Digital Subtraction Angiography machines with installed CBCT virtual navigation guidance software (XperCT and XperGuide software, AlluraXperFD20, Philips Healthcare, Best, The Netherlands). Therefore, PTNB procedures were changed to being routinely performed by CBCT guidance as of April 1, 2007.
Routinely, PTNB in our practice is performed using core needle biopsy technique only, due to the requirement of the pathologist for adequate tissue sampling and histological interpretation. Additional, immunochemistry special stains are also required in some cases; therefore, adequate tissue sampling is necessary. A co-axial cutting needle technique was used with a 20-gauge semi-automated cutting needle adjoined with a 19-gauge co-axial introducer needle (Bard biopsy, Tempe, Arizona, USA). After skin disinfection, local anaesthesia with 2% lidocaine was injected at the skin marker of the access site. A co-axial introducer needle was inserted from the marker site to the targeted pulmonary nodule. A cutting needle was then inserted into the co-axial introducer needle to remove tissue samples. Three pieces of tissue were routinely obtained and sent for histological analysis. After the procedure, all patients were closely observed in the observation room for outpatients or in the wards for inpatients for at least four hours. Chest radiography in an upright position was routinely performed four hours after the procedure to highlight possible late complications. However, in cases that had changes in vital signs or clinical status during the observation, chest radiography was repeated to determine the cause of complication. Emergency management was promptly undertaken for patients who had immediate, major complications.
Conventional computed tomography guidance
CCT was performed with a low-dose CT scan with the following imaging parameters: voltage of 100 kVp, tube current of 50 mA, rotation time of 0.4 second and collimation of 20 mm × 5 mm slice thickness. A pre-procedural chest CT scan was performed to establish the cutaneous access point marked on the patient’s skin. A co-axial introducer needle was advanced from the marker site until its tip was located in the subcutaneous tissue of the chest wall. A second chest CT scan was conducted to evaluate the precise plane, direction, and position of the needle tip before advancement of the co-axial needle into the target nodule. Each needle path into the lung was followed by repeated CT scans to confirm that the needle tip was correctly reaching the target nodule (Figure 1), upon which the biopsy was conducted. A post-biopsy chest CT scan was conducted to assess any complications of the procedure. The procedure was performed using the standard protocol of ‘move off and scan’ to prevent radiation exposure to the operator [13,14].
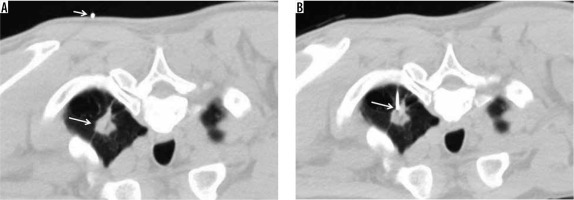
Figure 1
Conventional computed tomography-guided technique. A – Pre-procedural chest computed tomography (CT) showing spiculated mass at the apicoposterior segment of the left upper lobe (large arrow), with the access marker (small arrow) on the skin. B – Chest CT after insertion of the co-axial needle into the left lung; the tip of the co-axial needle being placed in the target nodule (arrow)
C-arm cone-beam computed tomography guidance
A pre-procedural CBCT scan was performed for planning the biopsy by using XperGuide software. Each targeted lung nodule was defined as a target point, whilst the skin marker was defined as an entry point. The software program was connected to the entry and target points to ensure the direction of the needle pathway (Figure 2). XperGuide software automatically displayed the needle’s ‘entry point’ on the patient’s skin seen as a ‘bull’s eye view’. This phase of the procedure followed by C-arm positioning was called ‘entry point positioning’. Under fluoroscopy, the operator positioned the needle tip at the cutaneous entry point on the basis of the CBCT image appearing on the monitor. At this point, the C-arm was rotated in the ‘progression view’ position, which is perpendicular to the initial C-arm position. The needle was advanced into the thorax following the virtual path, which was displayed on the monitor in real-time fluoroscopy, until it reached the target point. A secondary CBCT was then performed to confirm that the position of the needle tip was in the target nodule. After establishing the position of the tip, the biopsy was conducted. A final CBCT was performed to assess any biopsy-related complications.
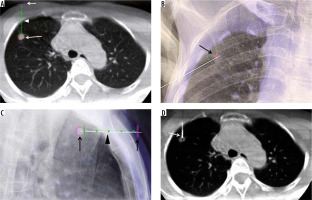
Figure 2
C-arm cone-beam computed tomography (CBCT)-guided technique with XperGuide software. A – Entry point view of CBCT showing the target nodule (large arrow), access point in the skin (small arrow), and the navigated pathway from the skin to the target nodule (arrowhead). B – Frontal view of fluoroscopy showing access marker on the right chest wall as a ‘bullseye appearance’ (arrow). C – Progression view of fluoroscopy showing co-axial needle from the access marker site (small arrow), along the navigated line (arrowhead) into the target nodule (large arrow). D – CBCT confirming the tip of the co-axial needle going into the target nodule
Data collection and definition
Data were collected from the hospital database and records of the Radiology Department. Patients’ demographic data, blood pressure, coagulation parameters, pulmonary nodule characteristics, position, radiation dose, procedural complications, and histological results were recorded.
Adequate tissue sampling was defined as that which was sufficient for histological diagnosis. Major complications were defined as those that required major therapy, prolonged hospitalisation of more than 48 hours, permanent adverse sequelae, or death. Minor complications were defined as those that did not require therapy or those just requiring observation [15]. Patient radiation doses were presented as an effective dose and were the sum of the dose equivalents for each organ in the body.
Statistical analysis
Quantitative parameters were presented as mean ± standard deviation, and qualitative parameters were expressed as counted numbers and percentages by using R software version 3.3.3. The diagnostic test was calculated by MedCalc statistical software. Categorical variables were compared using the Χ2 test, Fisher’s exact test, the rank-sum test, or t-test where appropriate. A p-value of less than 0.05 was considered as a statistically significant difference.
Results
A total of 266 and 100 nodules were biopsied with CCT and CBCT guidance, respectively. Patients’ demographic data are shown in Table 1. The mean age of patients in the CBCT guidance group was slightly higher than that in the CCT guidance group (64 years vs. 62 years; p = 0.081). The percentage of females was higher than that of males in both groups. No statistically significant differences were observed in the following: blood pressure, haemoglobin (Hb), platelet count, prothrombin time, and international normalise ratio between the two groups (p > 0.05).
Table 1
Demographic data of patients
The details of the pulmonary nodules, dose of patient radiation exposure, and complications after biopsy are presented in Table 2. No statistically significant difference in the mean diameter of nodules was found between the two groups (2.9 cm vs. 3.2 cm; p = 0.081). No statistically significant differences in the location of nodules or position of the patients during the procedures were detected between the two groups (p > 0.05). The mean radiation received by the patients in terms of effective dose was slightly higher in the CBCT guidance group, but the difference was not statistically significant. Most complications in both groups were considered minor. Thirteen patients in the CCT guidance group and three patients in the CBCT guidance group had major complications. In the CCT guidance group, 11 patients had large amounts of pneumothorax after biopsy, which required chest tube drainage. Two patients had stroke, with one-sided hemiparesis, immediately after the procedure. An emergency CT scan of the brain revealed no significant abnormality in both patients. Further magnetic resonance images showed focal areas of cerebral infarctions in both patients. In the CBCT guidance group, three patients had large amounts of pneumothorax after biopsy and required chest tube drainage.
Table 2
Characteristic of pulmonary nodules, position of biopsy, patient radiation exposure, and complications
The details of tissue sampling between the two groups are presented in Table 3. Statistically significant differences in adequate tissue sampling were detected between CCT and CBCT guidance groups (86.1% vs. 95%; p = 0.028). The number of nodules requiring re-biopsy was higher in the CCT guidance group (19.9%; p < 0.001). The histological results of 53 re-biopsied nodules in the CCT guidance group revealed 28 malignant and 25 benign lesions. The five nodules that were re-biopsied in the CBCT guidance group were malignant lesions. No statistically significant differences in the final histological results were found between the two techniques (p = 0.059).
Table 3
The results of tissue sampling
The sensitivity, specificity, and accuracy of CCT guidance for the diagnosis of malignancy were 84.1%, 100%, and 89.9%, respectively, whereas those of CBCT guidance were 93.3%, 100%, and 95.0%, respectively (Table 4).
Discussion
In this study, no significant differences were found in terms of demographic data and characteristics of pulmonary nodules between CCT and CBCT guidance groups. However, the mean radiation exposure in terms of effective doses was slightly higher in the CBCT guidance group. The rates of minor and major complications were similar between the two groups. The number of sufficient tissue samplings and the accuracy were significantly higher in the CBCT guidance group.
The mean estimated effective dose of CBCT guidance (5.6 mSv) was slightly higher than that of CCT guidance (5.4 mSv), but the difference was not statistically significant. The mean estimated effective dose of CBCT guidance in our study was within the range of 3.4–8.6 mSv as seen in recent reports [7-9,13,16]. The variation in the effective dose of CBCT guidance can be explained by differences in the protocols used in each institution. First, most studies including the present one usually conducted CBCT scanning, per case, three times; namely, pre-planning, intra-procedure, and post-procedural CBCTs [8,9,13,16]. Hwang et al. only performed two CBCT scans per case and did not conduct a post-procedural CBCT [7]. Second, some studies used high-dose mode to obtain high-quality images [8,9,16]. By contrast, Hwang et al., Cheng et al., and the present study used low-dose mode to reduce radiation exposure [7,13]. The results of CBCT guidance indicated a significant reduction in the radiation exposure of the patients compared with CCT scan of the chest [7-9,13,16]. The mean effective dose was 11.05 mSv [17]. The mean estimated effective dose of CCT guidance in the present study was also markedly lower than that of CCT scan of the chest. This finding can be explained by the low-dose mode used in the present work to perform CCT-guided biopsy of pulmonary nodules. In our opinion, we should reduce the radiation exposure during a procedure as much as possible while still maintaining the quality of images that we can interpret.
The first and second most common complications of PTNB were pneumothorax and haemoptysis, respectively. Uncommon complications were air embolism, chest pain, procedural-related death, and needle tract metastasis [7-16]. The rate of pneumothorax reported in literature ranges from 15% to 30% of CT-guided biopsies, while the proportion of PTNBs requiring treatment by chest tube drainage after CT-guided biopsies ranges from 1% to 14% [8,11,16,18-22]. In the present study, the rate of chest tube drainage was similar between CBCT- and CCT-guidance groups, with values of 3% and 4.1%, respectively, which are lower than the range reported in the literature [8,11,16, 18-21]. The incidence of haemoptysis in CT-guided biopsy is within 2.0%–3.9% [20,23]. However, Lee et al. reported that the rate of haemoptysis requiring bronchial embolisation is 1.3% due to massive bleeding [16]. In the present study, no patients after PTNB had haemoptysis requiring bronchial embolisation. However, two patients in the CCT guidance group had an acute stroke caused by air embolism. After retrospective review, we found that the location of pulmonary nodules in each case was very close to attaching to the branch of the pulmonary vein, resulting in a high risk of air embolism occurring when cutting the tissue. As such, we did not find any air embolism in the CBCT-guidance group. In our opinion, for patients who have nodules that are in contact with the adjacent vessel, CBCT-guided PTNB may be a safe option because XperGuide software offers pre-procedure planning and real-time needle advancement. This strategy can avoid any straying in the passage of the co-axial needle along with any misdirection of the cutting needle, which may injure the vessel.
Previous studies reported that the diagnostic accuracy for malignancy by CCT guidance is within the range 62-93% [24-28]. Meanwhile, the diagnostic sensitivity and accuracy for malignancy by CBCT guidance ranges from 90% to 95.8% and from 91.7% to 97%, respectively [7,10,11,29]. Lee et al. conducted a study with the greatest dataset by using CBCT-guided PTNB of pulmonary nodules in 1153 patients. The results revealed 95.7% sensitivity, 100% specificity, and 97% accuracy for the diagnosis of malignancy [16]. The results of CBCT-guided biopsy for pulmonary nodules in the present study are similar to previous reports [7,10,11,16,29]. Moreover, in our study, directional comparison indicated that the accuracy of CBCT guidance was slightly higher than that of CCT guidance. Our study and previous studies showed that CBCT-guided PTNB has superior accuracy over CCT-guided PTNB for pulmonary nodules [7,10,11,16,29]. The reason could be explained by the high success of navigated-guide software with real-time needle insertion in reaching the targeted lesion. Moreover, CBCT-guided PTNB has high accuracy in the biopsy of nodules located in the lower lobe or in elderly patients who have difficulty in controlling respiration [13]. By contrast, CCT guidance has lower accuracy because it uses a blind technique to insert the needle in targeting nodules.
The strength of this study is the direct comparison of CBCT and CCT guidance. Almost all of previous studies presented the results of CBCT-guided PTNB in one-arm results and indirectly compared it with CCT-guided PTNB from other studies. The present work also has several limitations. Firstly, this study had a retrospective design and was performed in a single centre. Further studies should adopt a prospective randomised design in multi-centres. Secondly, this study did not evaluate the risk factors of diagnostic failure or complications of each group.


