Introduction
With the development of modern imaging technologies such as computed tomography (CT) and magnetic resonance imaging (MRI), an increasing number of adrenal tumors, especially incidentalomas, have been detected [1, 2]. Laparoscopic adrenalectomy (LA) was first performed for adrenal tumors by Gagner et al. [3] in 1992 and has been gradually performed for resecting functional, non-functional, and malignant adrenal tumors, thereby replacing open surgery as the gold standard for managing adrenal tumors [4–6]. Laparoscopic surgery has shorter hospital stay, better trauma management, faster recovery, and lower costs than open surgery [5, 7]. US national surveys have also confirmed that LA has significantly lower perioperative morbidity than open adrenalectomy [8]. However, patients who have undergone previous abdominal surgery, especially kidney, liver, and spleen surgery, often have adhesions in the adrenal gland region, and perioperative risk is significantly increased during trans-peritoneal laparoscopic adrenalectomy (TLA) [9, 10]. Shortly after Gagner et al.’s study, Mercan and Mandressi proposed retroperitoneal laparoscopic adrenalectomy (RLA) as an alternative to TLA because it can directly and rapidly enter the surgical area without mobilizing intraperitoneal organs [11, 12]. However, there are still different perspectives regarding the treatment of adrenal tumors by TLA and RLA in published meta-analyses [13, 14].
The incidence rate of obesity is increasing annually, including morbid obesity caused by Cushing’s syndrome, which has become a global concern [3, 4, 15]. Obesity is often associated with high blood pressure, diabetes, and cardiovascular disease, which increases the risk of surgical complications such as adrenalectomy [16]. However, various studies have yielded conflicting results, and some studies have even found that obese patients may benefit from minimally invasive surgery [17, 18].
Nevertheless, to date, no relevant studies have confirmed the safety of LA in obese patients and determined the most beneficial surgical approach for obese patients. To fill this gap, this meta-analysis aimed to evaluate peri- and postoperative outcomes of LA in patients with adrenal tumors.
Aim
The aim of the study is to evaluate the efficacy and safety of laparoscopic adrenalectomy in nonobese versus obese patients in terms of perioperative outcomes and postoperative outcomes.
Material and methods
This systematic review and meta-analysis followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline [19].
Literature search and eligibility criteria
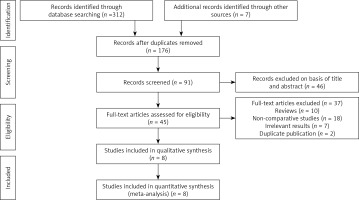
We systematically searched papers published in PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Science databases and Cochrane Library. The time range of the literature search was up until July 2021. No language restriction was applied. Search items included “laparoscopic adrenalectomy”, “adrenal tumor”, “body mass index (BMI)”, and “obesity”. Additionally, some research references were searched manually. Our meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Figure 1) [20].
The inclusion criteria of the study were as follows: (1) studies with randomized controlled trials (RCTs), case-control studies, or cohort studies; (2) patients with adrenal tumors requiring LA, including RLA or TLA; (3) comparing nonobese with obese patients; and (4) full articles containing at least one outcome parameter such as operation time (OT), estimated blood loss (EBL), length of hospital stay (LOS), open conversion rate, and total complications. The exclusion criteria were as follows: repetitive reviews, letters, case reports, studies unrelated to the subject matter, duplicated studies based on the same patients, or studies with no detailed data.
According to the above inclusion and exclusion criteria, the procedure was independently completed by three researchers and differences between investigators observed in the process were resolved through negotiation.
Data extraction
All relevant data were extracted by four authors independently. The researchers used standard tables to extract all collected interest outcomes for each included study: first author’s name, country, study design, sample size, intervention, age, and tumor size. Surgical outcomes included OT, LOS, EBL, conversion rate, and total complications. In addition, some of the data in the study were converted to a mean ± standard deviation, and the mean ± standard deviation of overweight with normal body weight was combined [21, 22].
Quality evaluation
Based on preliminary search results, the Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included studies [23]. According to the evaluation of the three question areas of selection, comparability, and exposure in the scale, a score of more than six stars can be considered to indicate high-quality research.
The above steps were completed by two of us (ZY.X and HL.L) independently. After discussion, the disagreements were resolved by the senior author (XD.L).
Statistical analysis
Review Manager Version 5.4, software (Cochrane Collaboration, Oxford, UK) and Stata 16 (StataCorp LP, University City, Texas, USA) were used for statistical analysis. The Q test and χ2 test were used to verify the heterogeneity between the included studies. According to the test results of heterogeneity, if I2 ≥ 50% or p < 0.1, the random-effect model was used for pooled estimates. Otherwise, fixed-effects models were used for the analyses. I2 > 50% indicates significant heterogeneity between studies. In addition, sensitivity analysis was performed to explore potential sources of heterogeneity. P < 0.05 was defined as statistically significant.
Results
Description of studies
In total, 319 records were retrieved from five databases. After reading the title and name of the first author, 143 duplicate studies were excluded. After further analyzing the abstract, research topics, and keywords, 46 records unrelated to the research topics were excluded. After reading the full text, 37 studies were excluded. Finally, seven retrospective studies and one prospective comparative trial with 1721 patients were included in the meta-analysis (Figure 1) [15, 24–30].
Baseline data, including the first author’s name, age, country, study design, and intervention model, are presented in Table I.
Table I
Basic characteristics and quality assessment of enrolled studies
| Study | Country | Design | Intervention | Age [years] | Tumor size | Approach | Quality scores |
|---|---|---|---|---|---|---|---|
| Nonobese (N)/Obese (N) | Nonobese/Obese | ||||||
| Inaishi et al. [29] | Japan | Retrospective comparative trial | 70/28 | 51.7 ±40.1/42.7 ±48.4 | 3.4 ±3.6/2.7 ±4.5 | Transperitoneal | 6 |
| Zonča et al. [30] | Czech Republic | Retrospective comparative trial | 96/41 | 51 ±15/53 ±14 | 5.4 ±2.3/4.7 ±1.6 | Retroperitoneal | 7 |
| Dancea et al. [26] | USA | Retrospective comparative trial | 31/49 | 56.1 ±14/50.1 ±13 | 4.3 ±2.0/4.5 ±3.1 | Transperitoneal | 6 |
| Rodríguez-Hermosa et al. [24] | Spain | Prospective comparative trial | 70/90 | 57.0 ±11.6/48.5 ±13.9 | 5.0 ±2.9/5.0 ±2.4 | Transperitoneal | 8 |
| Hu et al. [15] | China | Retrospective comparative trial | 290/63 | 48.88 ±12.35/46.11 ±11.83 | 2.74 ±1.30/2.67 ±1.49 | Retroperitoneal | 7 |
| Ortenzi et al. [28] | Italy | Retrospective comparative trial | 149/79 | _ | 4.3 ±1.9/4.3 ± 1.8 | Transperitoneal | 6 |
| Kazaryan et al. [25] | Norway | Prospective comparative trial | 133/39 | 48.8 ±43.4/55.3 ±43.9 | 5.2 ±7.6/2.7 ± 3.4 | Transperitoneal | 7 |
| Pędziwiatr et al. [27] | Poland | Retrospective comparative trial | 346/122 | 54.2 ±15.0/55.7 ±11.3 | 4.3 ±2.1/4.2 ±2.9 | Transperitoneal | 6 |
Quality assessment
Based on the NOS scoring rules, we have listed the final study quality scores in Table I.
Perioperative outcomes
Tumor size
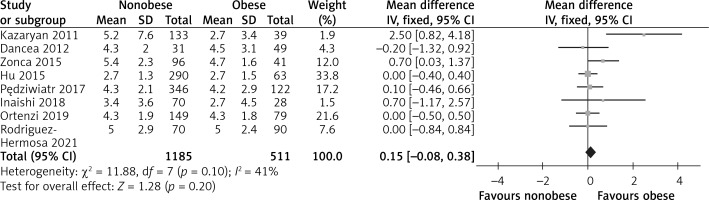
There were eight studies that reported tumor size [15, 24–30]. Due to the heterogeneity test outcome (I2 = 41%), we used a fixed effect model. The results showed that there was no significant difference between the two groups (WMD = 0.15, 95% CI: –0.08 to 0.38, p = 0.20, Figure 2).
Operation time
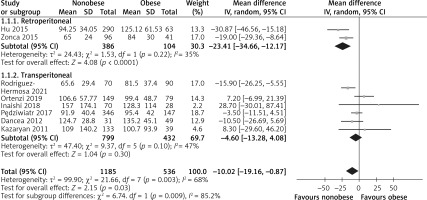
Eight studies reported the OT, and 1721 patients were enrolled [15, 24–30]. The heterogeneity among the studies was within the acceptable range (I2 = 68%, p = 0.003), and the random effects model was used. The meta-analysis results indicated that compared with the obese group, the nonobese group had shorter OT, and the difference between the two groups was statistically significant (WMD = –10.02, 95% CI: –19.16 to 0.87, p = 0.03, Figure 3). Moreover, subgroup analysis showed that a shorter OT was related to the retroperitoneal approach in the nonobese group (WMD = –23.41, 95% CI: –34.66 to –12.17, p = 0.0001, Figure 3).
Estimated blood loss
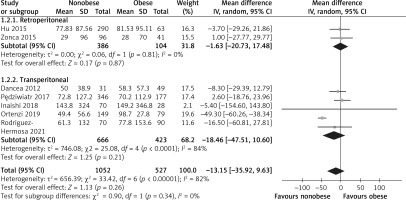
Seven studies reported on EBL and involved 1579 patient samples [15, 24, 26–30]. The heterogeneity among the studies was relatively high (I2 = 82.0%, p = 0.00001), and the random effects model was applicable. No significant difference was observed between the two groups (WMD = –13.15, 95% CI: –35.92 to 9.63, p = 0.26, Figure 4). According to the subgroup analysis, there was no significant difference in EBL in either RLA or TLA between the obese and nonobese groups (RLA (WMD = –1.63, 95% CI: –20.73 to 17.48, p = 0.87), TLA (WMD = –18.46, 95% CI: –47.51 to 10.6), p = 0.21, Figure 4).
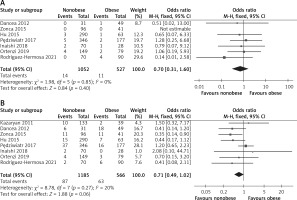
Conversion rate
Seven studies reported conversion rate, and a total of 1579 patients were enrolled [15, 24, 26–30]. Due to the heterogeneity test outcome (I2 = 0%), we used a fixed effect model. The results of the meta-analysis revealed no significant difference between the two groups (OR = 0.70; 95% CI: 1.31 to 1.60; p = 0.40, Figure 5 A).
Postoperative indicators
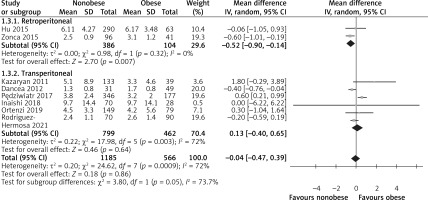
Length of hospital stay
LOS was recorded in eight studies [15, 24–30]. Because of significant heterogeneity among the studies (I2 = 72%, p = 0.0009), the random effects model was used to combine the results. According to the meta-analysis results, the difference between the two groups was not statistically significant (WMD = –0.04, 95% CI: –0.47 to 0.39, p = 0.86, Figure 6). The subgroup analysis showed that the LOS of RLA was shorter in the nonobese group than in the obese group (WMD = –0.52, 95% CI: –0.90 to 0.14, p = 0.007, Figure 6), but no significant difference was observed between the two groups with respect to TLA (WMD = 0.13, 95% CI: –0.40 to 0.65, p = 0.64, Figure 6).
Perioperative complications
In the statistical analysis, the complication data were obtained from 8 studies [15, 24–30] (Figure 5 B). Because of low heterogeneity (I2 = 20%), we used a fixed effect model. No significant difference between the nonobese and obese groups was found (OR = 0.71; 95% CI: 0.49 to 1.02; I2 = 0%; p = 0.06).
Sensitivity analysis
Based on the high heterogeneity of OT, EBL, and LOS, we performed a sensitivity analysis to determine the effect of each study’s data on the final outcomes. Studies were removed one by one, and we found that OT and LOS were stable (Figures 7 A, C). However, EBL in Ortenzi et al.’s study was possibly the cause of the high heterogeneity in the EBL index (Figure 7 B).
Discussion
LA has been considered a substitute for traditional open surgery in the past decade by numerous studies [5–8]. Moreover, a study showed that laparoscopy was also safe and feasible for the treatment of large adrenal tumors [31]. However, the increase in the incidence rate of obesity, which is generally considered a major factor for increasing complications, particularly wound and septic complications [30], poses new challenges to LA. In addition, LA (including retroperitoneal or trans-peritoneal approaches) remains controversial in obese patients. Although a recent meta-analysis based on the results of five studies performed by Danwang et al. showed that obesity was not associated with surgical complications [18], this meta-analysis did not provide a comprehensive analysis of perioperative results and did not determine the clinical benefits of LA in obese patients. Based on the above reasons, we performed the current meta-analysis to analyze perioperative results of LA in obese and nonobese patients.
We performed an evidence-based medicine analysis of eight published studies that explored peri- and postoperative outcomes of LA in obese patients. Our results were consistent with those of previous studies [18, 24, 29], which found no difference in most perioperative outcomes, except OT, and postoperative outcomes between obese and nonobese patients who underwent LA. Some studies also found that obesity did not result in worse outcomes in patients undergoing other minimally invasive procedures [16, 32, 33].
Regarding OT, our meta-analysis showed that the nonobese group had shorter OT than the obese group, which was consistent with the results of several previous studies. However, in the subgroup analysis, we found that the OT of obese patients who underwent TLA was not prolonged, but the OT of nonobese patients was slightly shorter than that of obese patients when RLA was performed. Kazaryan et al. also found that OT was moderately higher in obese patients than in nonobese patients, and it had a weak but significant correlation with BMI [25]. The most significant advantage of retroperitoneal endoscopy is that the endoscope enters the gland directly (especially when using the dorsal approach), while the massive fat around the gland impairs exposure and the right adrenal gland is partially retrocaval and drains directly to the inferior vena cava through a short central vein, making it more difficult for surgeons to operate, thus prolonging OT [25, 34]. There are also inconsistent reports in the literature. Some authors have confirmed the feasibility of LA in obese patients and revealed that BMI may have no effect on OT [24, 27, 28]. This could be explained by the three-dimensional vision system, which could improve the depth of perception, and some surgeons usually place additional trocar(s) when trying to obtain a good surgical field of vision. Furthermore, the introduction of energy devices may minimize the difficulty of hemostasis in heavy adipose tissues [29, 35]. In addition, the operator’s experience and previous history of abdominal surgery were interfering factors [24].
Blood loss has always been a major concern among clinicians. In our meta-analysis, the EBL of the two groups was not statistically different. However, the heterogeneity of the results was high. Sensitivity analysis showed that the heterogeneity may be because of the use of different hemostatic devices by Ortenzi et al., but ultrasonic and clip hemostasis methods are not effective in obese patients [28]. In addition, subgroup analyses indicated that both TLA and RLA in the nonobese and obese groups had similar EBL. It seems that there is a difference between this and our conventional ideas. However, the size of the surgical wound is small in both groups, and sufficient extra space in the abdominal cavity is created by the pneumoperitoneum during laparoscopy so that the operator can thoroughly stop the bleeding [25]. The application of good energy devices is also a reason for reduced bleeding [29]. Certainly, laparoscopic surgery is performed by a team of experienced surgeons.
According to our meta-analysis, seven articles reported the conversion rate, and no significant difference between the nonobese and obese groups was found (OR = 0.70, 95% CI: 1.31 to 1.60, p = 0.40, Figure 4 A). According to literature reports, open conversion is often related to obesity, intraoperative bleeding, adhesion, excessive tumor size, and inexperienced doctors [36, 37]. Most studies have also shown that there is no significant correlation between obesity and intraoperative conversion to open surgery [15, 24–28, 38, 39]. The following factors may further explain these results. First, there is a certain difference between obesity estimated by BMI and visceral fat mass in patients; even if BMI is in the normal range, its high visceral fat content affects postoperative outcomes [40]. Second, most conversions to open surgery occur in the learning curve. Third, conversion may be mainly affected by blood loss and subsequent poor outcomes [29, 41].
We confirmed that there is no significant difference in the LOS between the two groups, which is consistent with the outcomes of all TLAs included in the literature. The opposite result was obtained in the subgroup analysis: the LOS of obese patients after RLA was longer than that of nonobese patients because of the small sample size and because TLA is usually adopted by surgeons as the first choice during the procedure. Longer LOS might have resulted owing to the higher number of postoperative complications and the lower ability of obese patients to recover from surgery. According to the literature, obese patients have a high American Society of Anesthesiologists (ASA) score and a corresponding increase in the risk for cardiovascular complications, pulmonary insufficiency, and pneumonia [15]. Therefore, even if it is a minimally invasive technology, its effect on the functional recovery of patients after surgery cannot be ignored.
Regarding overall complication rates, our meta-analysis did not show any specific differences between the two groups. Based on a recent subgroup analysis of Clavien-Dindo grade classification of complications [18], we did not observe any statistically significant difference. Obese patients may have higher ASA scores than nonobese patients. However, multivariate logistic regression analysis showed that BMI could not be considered an independent risk factor for postoperative complications [15]. In contrast, the methods to assess complications have been continuously amended, and points of indications have also been revised. These factors may obscure the effect of obesity. However, Dancea et al. found that obese patients have relatively high postoperative complication rates. Because of the heavy burden on the greater omentum in obese patients, surgeons prefer to mobilize the splenic flexure to reach the left adrenal gland, which can easily damage the spleen during left-sided TLA, and 4 cases of spleen damage were observed in this cohort [26]. A poor intraoperative visual field in obese patients, previous abdominal surgery, and lack of operator’s experience are also factors for complications [24, 41].
Our meta-analysis strictly followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines [42]. However, this study had some limitations. First, most of the included studies were primarily retrospective, and the level of evidence in evidence-based medicine was not sufficiently high; thus, the conclusions of this meta-analysis need to be treated with caution. Second, few articles included the study of RLA for obese patients, and there may have been selection bias. Third, the number of included studies was relatively small. Finally, significant heterogeneity existed in some evaluation indices.
Conclusions
Based on current evidence, this meta-analysis suggests that the LA is feasible and effective in patients with obesity. But the conclusions need to be treated with caution due to high heterogeneity. This conclusion needs to be verified by a prospective cohort study with a larger sample size and a more rigorous design.