Introduction
Pathologies of the ascending tract of the thoracic aorta are often life-threatening.
A multitude of surgical techniques are used to repair damaged aorta, such as the Wheat procedure (consisting of placing an aortic graft and an aortic valve prosthesis), Bentall and Cabrol approaches (by placing a composite artificial graft), Ross intervention (implanting a biological aortic graft), David technique and its derivatives (allocating an aortic graft without any intervention on the aortic valve), and elephant trunk (arch repair).
A proper imaging computed tomography (CT) protocol facilitates a correct diagnosis.
In this article, we discuss the main diseases affecting the ascending aorta and the different surgical techniques used to treat them. We also discuss the computed tomography imaging findings before and after surgery, including some of the most common complications.
Discussion
Pathological conditions
Aortic aneurysm
Aneurysm of the aorta is a focal and persistent enlargement of all aortic wall layers greater than 50% compared to a normal calibre [1].
The thoracic aorta is made up of the aortic root, ascending tract, aortic arch, and descending section. The ascending segment spans from the root to the origin of the right brachiocephalic artery; the arch, from the right brachiocephalic artery to the attachment of the ligamentum arteriosum; and the descending section, from the ligamentum arteriosum to the aortic hiatus of the diaphragm. The aortic root comprises the valve, annulus and sinuses [2].
A frequent cause of aneurysm of the ascending segment is caused by cystic medial degeneration; in this condition, looking at histology samples, muscle cells are less represented and loss of function of the elastic fibres is noted.
This process leads to aneurysm of the vessel, by strength loss of the wall layers [3].
Clinical studies describe vastly different growth rates of the aneurysms of the ascending tract of the aorta spanning from 0.07 cm/y to 0.42 cm/y. As the dilatation grows, so does the risk of life-threatening events; in patients with a 6 cm aneurysm, risk of rupture, dissection or death reaches 14.1% [4]. In some associated diseases, such as Marfan [4], Loeys-Dietz syndrome [5], and abnormal congenital aortic valve (such as bicuspid valve), surgery should be performed earlier. In particular, a diameter of 4.5 cm or greater is considered as a cut-off for surgical operation [6].
Other causes of aortic enlargement and aneurysms are large vessel vasculitis, for example Takayasu arteritis [7].
Aortic dissection
The aortic wall is made up of 3 stratums (from the innermost to the outermost): intima, media, and adventitia.
Longitudinal laceration of the aortic wall, allowing blood to flow between the intima and the adventitia, dividing the media layer, is called dissection [8]. In this condition 2 lumina are created, a false and a true lumen [9].
Because of the superior pressure inside the false lumen, the true lumen is smaller and may be compressed or obstructed. The false lumen may remain patent, close due to thrombosis, unite with the true lumen distally, or rupture, allowing blood to spill into nearby structures such as the pericardial, pleural, or peritoneal cavities. If the dissection extends to side branch arteries, such as cerebral, coronary, mesenteric, or renal, ischaemia is a probable occurrence due to reduced blood flow to these districts [10].
Aortic dissection is currently classified using the Stanford classification, which includes 2 types based on the required surgical intervention: A and B. The former is the most frequent, accounting for 75% of cases [11]; in type A, dissection involves the ascending aorta or both the ascending and aortic arch or also descending aorta; acute type A dissection represents an emergency condition due to potentially fatal complications, such as involvement of the coronary arteries, the aortic valve, or rupture into chest cavities such as the pleural space or the pericardium (hence cardiac tamponade), warranting prompt surgical intervention.
Chronic type A dissection is also treated surgically; often, in this condition, medial layer disease is present (such as cystic medial necrosis).
Stanford type B is dissection of the aorta originating distal to the left subclavian artery. Usually this type does not require surgical intervention, and medical treatment is the option of choice unless signs of progression of the disease arise (for example, persistent abdominal pain, suggestive, in the absence of other causes, of bowel ischaemia) [11].
Surgical approaches and computed tomography imaging findings
Throughout the years different surgical repair techniques have been invented. Some examples are reconstruction of the ascending aorta by using a graft with (Wheat procedure) or without a prosthetic valve; reconstruction with a composite artificial graft (Bentall and Cabrol approaches), or placing a biological graft (Ross intervention and homograft reconstruction); rebuilding of the ascending aorta with a composite graft, retaining the patient’s valve (David technique and derivatives); arch repair (elephant trunk intervention).
In almost all surgical techniques the patient’s damaged aorta is removed and a graft (whether biological or artificial) is implanted. Nowadays the graft is connected to the patient’s undamaged vessel, because it always gives better results than the previous method, where the graft was positioned within the damaged aorta – a technique called inclusion graft [12].
Synthetic grafts are frequently made of polyethylene (Dacron). At baseline CT, they have slightly higher density than the native aortic wall [13].
After administration of intravenous contrast material, grafts may not be identifiable on the CT images, due to similar attenuation values with the contrast agent. The felt ring, instead, is slightly denser because of its thickness, and is thus recognisable. It is crucial to identify these structures because they may mimic pathologic conditions, such as pseudoaneurysms. For this reason, acquisition of both baseline and angiographic phase CT are advised in these patients [13] (Figure 1). An ECG-gated protocol allows the reduction of motion artifacts together with the evaluation of coronary arteries.
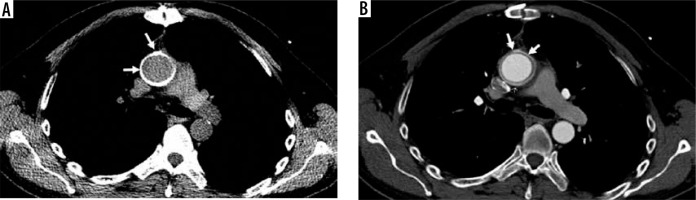
Figure 1
Aortic graft in the ascending aorta. Computed tomography scan (axial view) of an aortic graft in the ascending aorta: A) before contrast administration (best delineating the well-allocated graft, arrows) and B) after contrast administration (the graft is less distinguishable, arrows)
After surgery the usual imaging findings may consist of perigraft fluid collections and mediastinal air; fluid collections do not show any enhancement on contrast CT and are reabsorbed over time, not prompting intervention is required [14] (Figure 2).
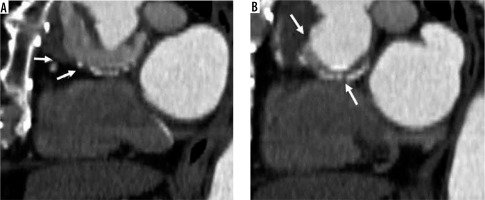
Figure 2
Perigraft fluid. Sagittal view, post-contrast computed tomography scan showing perigraft fluid (A and B, arrows)
However, if contrast enhancement is noted, or fluid collection increases in volume on the follow-up CT, an infection should be suspected. Moreover, postoperative mediastinal air is another usual finding [13]; persistent or new perigraft air raises the suspicion of anaerobic bacterial infection or formation of a fistula with the adjacent structures such as bronchus or oesophagus [14].
Coronary ostia sparing grafts
In 1964, Wheat and his pears performed successfully an intervention in which an aneurismatic ascending aorta was excised, the coronary ostia with a small part of the annulus retained, and the patient’s aortic valve was concurrently substituted. Since then this approach has been known as the Wheat procedure [15].
Sparing of the coronary ostia (that are anastomosed with the graft) reduces the risk of thrombosis, stenosis, kinking, and pseudoaneurysms at the coronary anastomosis spot [15].
One possible complication of this technique is dissection or aneurysm of the native aorta proximal to the graft. Some comorbidities increase the risk of these events, such as patients with annuloaortic ectasia or Marfan syndrome [12].
Composite and biological grafts
In 1968, Bentall and De Bono [16] conceived a procedure in patients with enlargement of both the ascending aorta and the aortic root. In this technique, the ascending aorta and the aortic root are removed and replaced by a graft. The coronary ostia are attached to the graft, and the patient’s aortic valve is taken out and replaced by a prosthetic one (Figure 3).
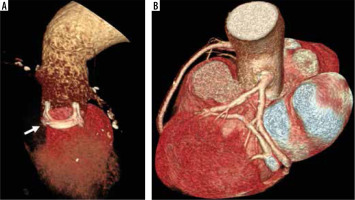
Figure 3
Bentall procedure. 3D volume rendering computed tomography images show Bentall procedure with a prosthetic valve implantation (A, arrow) and anastomoses of the coronary ostia to the graft (B)
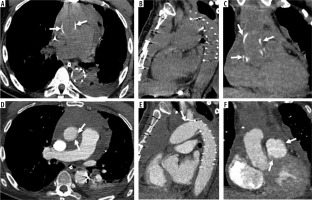
Frequent complications at the site of the coronary ostia attachments (kinking and thrombosis) warranted a new approach, known as “modified Bentall” or the “button Bentall” or “Carrel patch”. In this intervention, a round portion of the aorta surrounding the coronary ostia (the “button”) and the coronary arteries are excised. This allows easier reimplantation of the of coronary arteries to the graft [17] and a meaningful drop in complications, as mentioned in a retrospective study by Milano et al., in which out of 71 patients only 4 (6%) encountered complications [18] (Figure 4).
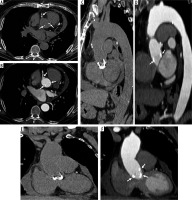
Figure 4
Modified Bentall procedure. Computed tomography (CT) scan showing a patient with modified Bentall procedure with pericardial effusion (A – axial, C – sagittal, E – coronal CT scans, before and B – axial, D – sagittal, F – coronal, after intravenous contrast administration)
These techniques, often performed by surgeons, present some drawbacks, such as the formation of pseudoaneurysms distal to the anastomoses site and dissection (Figures 5 and 6).
Figure 5
Complicated modified Bentall procedure. Patient with a history of modified Bentall procedure (30 years earlier). Computed tomography scans (A, B, C, G unenhanced and D, E, F, H after intravenous contrast administration) show ascending aorta aneurysm with mechanical aortic valve prosthesis, vascular prosthesis disconnection, and residual intimal flap due to dissection distal to the site of anastomosis
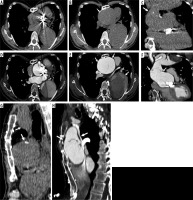
Figure 6
Modified Bentall procedure with aneurysm and distal aortic dissection. Male, 55 years old, with a history of modified Bentall procedure (10 years earlier). Axial enhanced computed tomography scan shows periprosthetic leak with aneurysm of the right coronary ostium (A, arrow), and distal aortic dissection (B – intimal flap, arrowhead) with wide false lumen
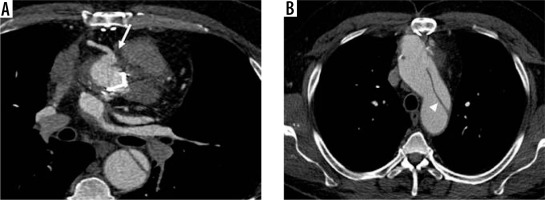
In 1981, another technique was developed – the Cabrol procedure – in order to offer an alternative approach to patients with aortic dissection or annuloaortic ectasia [12]. In the Cabrol technique, the native aortic valve and ascending aorta are removed and a new valve and a synthetic graft are placed; the coronary ostia are attached to prosthetic conduits and the latter are then connected to the aortic graft [19].
Some complications consist of anastomotic stenosis, periprosthetic hematoma and pseudoaneurysm [19,20].
Modified Bentall procedure is generally preferred [20].
Biological grafts are instead often used in young patients; in the Ross intervention, the patient’s pulmonary root replaces the native aortic root and an allograft is used to connect the right ventricle to the pulmonary artery [21], the main advantage is that anticoagulant therapy is not needed. The disadvantages include the duration of the valve (needing replacement after 10-20 years), complexity of the surgery, and potential complications to both valves (pulmonary and aortic) [21] and pseudoaneurysms located at both attachment sites [22].
Aortic valve-sparing techniques
Since artificial aortic valves require life-long anticoagulation therapy, other surgical repair procedures have been developed in order to preserve the native aortic valve while repairing a damaged ascending aorta [23]. Two main examples of valve-sparing aortic root repair (V-SARR) include the remodelling technique by Yacoub and the reimplantation procedure by David [24,25].
The former consists of asportation of the aneurysmatic aorta to the annulus level, keeping the native aortic valve in place and reconstructing the sinuses of Valsalva by inserting an artificial graft [24].
In the David technique the ascending aorta is removed, a graft is attached below the native valve, and the latter is introduced inside the graft [25]. In both techniques the coronary arteries are reimplanted into the aortic graft (Figure 7).
Figure 7
David procedure in Marfan syndrome. Axial and sagittal computed tomography images (A, B – before and C, D – after intravenous contrast administration) show David procedure in a patient with Marfan syndrome. C – shows aortic native valve (arrows)
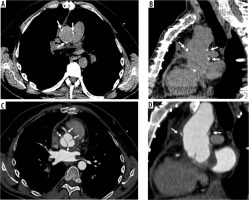
Some modifications of these technique have been invented, such as the T. David-V technique; in this procedure, the sinuses of Valsalva are reconstructed using a larger artificial graft. In the Demers and Miller intervention, 2 aortic grafts are connected together to create new sinuses; these are even wider than the ones of the T. David-V technique and allow the patient’s valve to be better attached within the artificial sinuses [26].
The main and most important complication of every aortic valve-sparing procedure is aortic valve insufficiency [23].
Arch repair
In 1983 Borst et al. invented another procedure to repair ascending thoracic aortic aneurysms: the elephant trunk [27].
This procedure consists of two stages; in the first one, a median sternotomy is performed, the aneurysmatic ascending aorta is removed, and an artificial graft is placed within the proximal descending aorta [28]. Three anastomoses are created: a proximal one in the ascending aorta, one with the brachiocephalic vessels, and a distal one after the origin of the left subclavian artery. The distal end of the graft is not connected to any structure and is free to move inside the lumen of the descending aorta.
In the second stage, another graft is placed inside the descending aorta and anastomosed to the previous one through a left thoracoabdominal access [28].
As in the previously mentioned techniques, some variations exist.
In 2003 the ‘frozen elephant trunk’ technique was introduced. This intervention modifies the second stage of the elephant trunk surgery, where a stent is introduced inside the descending aorta lumen through a femoral artery access, thus removing the need for a second open surgery and lowering mortality rates [29-32] (Figures 8 and 9).
Figure 8
Frozen elephant trunk procedure. Axial and coronal computed tomography images (A, B, C before and D, E, F after intravenous contrast administration) show frozen elephant trunk procedure
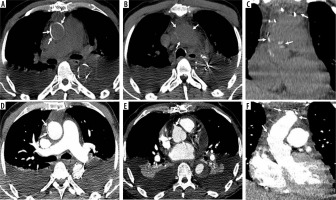
Figure 9
Frozen elephant trunk hybrid procedure. Axial, coronal, and sagittal computed tomography images (A, B, C before and panel D, E, F after intravenous contrast administration) show frozen elephant trunk hybrid procedure. The grafts are well allocated on panel A and C, and there are no peri-prosthesis leaks on panel D, E, and F
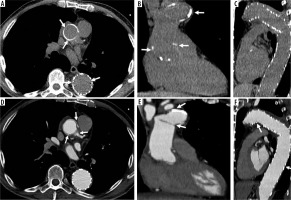
The most common complications are laceration (and rupture) at the distal anastomosis site, air embolism, recurrent left-sided laryngeal nerve injury, and hence vocal cord paralysis, graft kinking and occlusion, haemorrhage, neurological dysfunction in case of hypoperfusion of the brachiocephalic vessels (such as encephalopathy or stroke), and death from rupture of the aneurysm, tamponade, or sepsis [33] (Figure 10).
Figure 10
Elephant trunk hybrid procedure with hemopericardium. Axial, sagittal and coronal computed tomography images (A, B, C before and D, E, F after intravenous contrast administration) show elephant trunk hybrid procedure complicated with loculated mediastinal effusion and hemopericardium (A, D)
Conclusions
A variety of surgical techniques are used to repair a damaged ascending thoracic aorta. It is important for the radiologist to know the type of technique used and to recognize the normal structures of the grafts, in order to be able to spot the imaging features associated with the complications of these procedures.


