Introduction
Degenerative cervical radiculopathy is a neurologic disease characterized by dysfunction of the cervical spinal nerve, its roots, or both. Typically, affected patients present with radicular pain in the arm and neck, with possible hypoesthesia or weakness in the affected nerve-root distribution area. Usually, degenerative cervical radiculopathy is caused by an intervertebral foramen narrowing with encroachment of neural structures by various factors, including osteophyte formation and posterolateral cervical disc displacement [1]. The annual incidence rates of cervical radiculopathy in the USA are 107 per 100,000 men and 64 per 100,000 women [2]. Cervical radiculopathy risk increases with aging, and thus its incidence is expected to increase in the coming decades due to population aging. Neck pain and low back pain were ranked the 4th leading causes of disability-adjusted life years globally in 2015 [3]. The growing needs of the patient population affected by degenerative radiculopathy underscore the need for spine surgeons to provide practical and financially sound treatment options.
Nonsurgical protocols for degenerative cervical radiculopathy have included analgesia, translaminar and transforaminal epidural injections of corticosteroid, short-term immobilization, and cervical traction. When nonsurgical treatment plans fail, physicians turn to surgical approaches with favorable risk-benefit ratios. Advances in neuroimaging and minimal access techniques, such as endoscopy, digital video quality, and percutaneous systems, have enabled rapid improvement in and popularization of minimally invasive spinal surgery for the treatment of degenerative cervical radiculopathy [4, 5].
Percutaneous endoscopic cervical foraminotomy and discectomy (PECD) is an essential minimally invasive spinal surgery technique that has been adopted gradually by surgeons owing to its advantages of being minimally invasive, economical, and conducive to a rapid recovery. Posterior PECD (P-PECD) has become a valuable alternative operative approach to traditional open procedures [6]. Many clinicians who care for patients with cervical radiculopathy are uncertain about the efficacy and safety of endoscopic surgery. Furthermore, the absence of an intraoperative three-dimensional (3D) image of the surgical field in P-PECD and its steep learning curve limit its use. The O-arm, a new-generation mobile CT scanner, has been used to address these challenges. It is a multi-dimensional surgical imaging platform with an “O” shaped gantry that has been reported to provide high-quality intraoperative imaging information to the surgeon [7]. However, clinical data regarding its impact in P-PECD are lacking.
Aim
In this study, we report on the clinical efficacy and safety of O-arm assisted P-PECD in patients diagnosed with degenerative cervical radiculopathy.
Material and methods
All P-PECD procedures performed in patients with degenerative cervical radiculopathy performed at the clinic or inpatient department of our center between January 2013 and January 2018 were reviewed retrospectively. Prior to the operation, all of the patients had undergone continuous conservative treatment (analgesia, physical therapy, and/or corticosteroid injection therapy) and had a history of cervical radiculopathy for at least 12 weeks with confirmatory cross-sectional imaging showing disc herniation or foraminal stenosis that corresponded with their clinical symptoms. The exclusion criteria were: cervical instability; free intervertebral disc; severe calcification of herniated disc tissue; central cervical spinal stenosis; cervical deformity; and non-tolerance of surgery due to a systemic disease or mental disorder.
All patients underwent routine preoperative cervical X-ray examinations in the anteroposterior and lateral positions during hyperextension and hyperflexion as well as cervical 3-dimensional computed tomography (CT) and magnetic resonance imaging (MRI). Additionally, spinal stability, disc herniation type, and interlaminar width were assessed. The entry points and trajectory of the working cannula were also delineated in the imaging workstation, and the corresponding parameters were recorded.
All surgical procedures were performed by a senior surgeon using the TESSYS system (Joimax GmbH, Germany). The patient was placed in a prone position under general or local anesthesia. An O-arm (Medtronic Sofamor Danek, Inc., Memphis, TN) was brought into the surgical field to confirm the diseased spinal segments based on O-arm fluorescence imaging. After routine disinfection and draping (Photo 1), the skin and subcutaneous tissue at the point of entry, which was approximately 1.5 cm lateral of the midline (ipsilateral to the operative target), were infiltrated by 1% lidocaine. An 18-gauge spinal needle was inserted to a predetermined depth along the planned preoperative path. Lateral fluoroscopic images of the O-arm were used to confirm that the puncture needle tip was located at the lateral mass of the target cervical level, and then a guide wire was introduced to dock on the bone. Then, an approximately 1.5 cm skin incision was made, and a series of dilators were inserted sequentially through the neck soft tissue. Lastly, the working cannula was placed in the desired position and 3D images of the diseased segments and working cannula were obtained by O-arm scanning (Photo 2). With the working cannula set in the desired position, endoscopic bipolar cautery and micro-forceps were used to remove the remaining muscle overlying the bone. The position of the working cannula was rechecked by the O-arm upon clear exposure of the margin of the inferior lamina, superior lamina, and medial junction of the facet joint (V-point).
Photo 1
Installation of an O-arm system in the operating room for P-PECD. The surgeon had good access to the patient’s cervical spine, which is positioned in the center of the coil (A). Following sterile draping of the O-arm (B), intraoperative CT scanning was performed
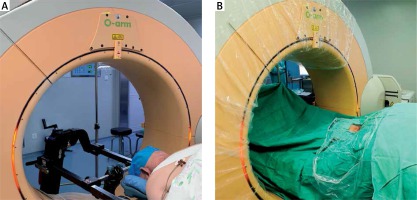
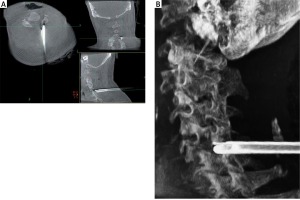
Photo 2
Intraoperative O-arm imaging to assist with working cannula placement. A – Intraoperative O-arm image of the cannula docked in the cervical lamina (axial, coronal, and sagittal views). B – 3D-reconstruction image used to demonstrate the position of the working cannula
Endoscopic punch and high-speed drills were used alternately to perform a lamino-foraminotomy, removing the outer cortical layer and cancellous bone of the lamina as well as the medial aspect of the facet. A small opening was made through the deep cortical bone over the lateral canal, through which an endoscopic rongeur was introduced to resect the ligamentum flavum and expand the decompression scope. The lamino-foraminotomy was performed after the nerve root had been well exposed along its proximal foraminal course. Briefly, after a small opening was made through the deep cortical bone over the lateral canal, the foraminotomy was completed with an endoscopic punch. At this stage, the medial and cephalad margins of the pedicle were identified for medial/lateral orientation. The foraminotomy was extended laterally until the lateral margin of the pedicle detached, at which point only approximately 30% of the medial facet (maximum limit of 50%) was removed, thereby conserving stability. Lateral nerve root decompression was completed when the lateral aspect of the cervical pedicle could be palpated with a small nerve hook. Thorough decompression was confirmed by O-arm imaging (Photo 3). The surroundings of target nerve roots were explored with a nerve hook. In cases involving a cervical herniated disc, the disc fragment compressing the nerve was removed to decompress the nerve roots fully (Photo 4). After hemostasis, the endoscopic system was removed, and the skin incision was glued closed.
Photo 3
Intraoperative O-arm images after decompression. Multiplanar images were used to check the adequacy of decompression and to evaluate facet joint preservation
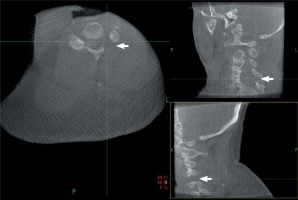
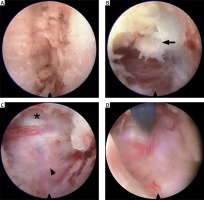
Photo 4
Intraoperative pictures of P-PECD. Following clear exposure of the V-point (A), foraminotomy was performed with a diamond drill. After ligamentum flavum (B, arrow) and bone removal, the thecal sac (C, asterisk) and nerve root (C, arrowhead) were identified. In cases of soft disc herniation, the ventral region of the nerve root was explored by micro-bipolar or nerve hook (D)
We assessed pain severity with the visual analogue scale for the neck (Neck-VAS) and arm (Arm-VAS) and assessed neck-specific quality of life with the neck disability index (NDI) at 1 month, 3 months, 6 months, and 12 months postoperatively [8]. Odom’s criteria were applied to evaluate the clinical outcomes at the last follow-up [9]. Follow-up data were obtained during clinic visits or by telephone interviews. Demographic, clinical, and surgical data were reviewed retrospectively. Continuous data are expressed as means ± standard deviations.
Results
During the 5-year study period (January 2013–January 2018), 36 consecutive patients underwent P-PECD with intraoperative O-arm imaging. From this source population, 32 patients with a mean follow-up of more than a year were included in this study. The demographic and clinical characteristics of the patient sample are reported in Table I together with average operating time, postoperative hospital stay duration, and follow-up period length data.
Table I
Demographics for P-PECD using intraoperative O-arm (n = 32)
The P-PECD operation was completed successfully in 32/32 patients (100%); no cases were transferred to open surgery. Intraoperative blood loss was < 10 ml in all cases. Intraoperative O-arm imaging compelled further decompression of remnant foraminal stenosis in 12/32 patients (37.5%). In the immediate postoperative period, patients reported only mild operation-related neck pain that was not of a severity requiring pain medication and that resolved within 3 days.
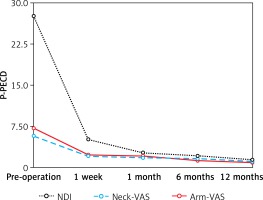
The clinical outcome data are shown in Table II and Figure 1. Note that compared with preoperative values, dramatic reductions in mean NDI, neck-VAS, and arm-VAS scores were achieved by 1 week postoperatively. NDI scores showed an additional substantial reduction from 1 week to 1 month postoperatively, after which NDI scores remained low. Meanwhile, neck- and arm-VAS scores remained low without further substantial changes throughout the follow-up period. According to Odom’s criteria, excellent postoperative efficacy was achieved in 14/32 patients (43.8%), good postoperative efficacy was achieved in 13/32 patients (40.6%), fair postoperative efficacy was obtained in 3/32 patients (9.4%), and poor postoperative efficacy was obtained in 2/32 patients (6.2%).
Table II
Clinical outcomes of P-PECD
| Variable | Pre-operation | 1 week | 1 month | 6 months | 12 months |
|---|---|---|---|---|---|
| NDI | 27.6 ±10.5 | 5.1 ±4.3 | 2.7 ±2.5 | 2.1 ±1.7 | 1.4 ±0.8 |
| Neck-VAS | 5.8 ±1.7 | 2.1 ±1.7 | 1.8 ±1.1 | 1.6 ±0.8 | 1.1 ±0.8 |
| Arm-VAS | 7.2 ±2.3 | 2.3 ±1.9 | 2.1 ±1.3 | 1.3 ±1.1 | 0.9 ±0.6 |
No permanent complications were observed during the surgical procedure or in the immediate postoperative period (such as cerebrospinal fluid leakage, infection, and hemiplegia). One patient, in whom good efficacy had been achieved, had a temporary postoperative complication. In this case, the spinal needle had been inserted errantly into the spinal canal during the establishment of the working trajectory. The patient experienced only upper limb weakness after the operation, which resolved by the 3-month follow-up.
Discussion
Various effective surgical interventions for cervical radiculopathy pain have been developed since Mixter and Barr first described the relationship between radiculopathy pain and degenerative spinal changes in 1934 [10]. The main objectives of these procedures are to remove pathogenic tissue, relieve nerve compression, and prevent recurrence. Until recently, lateral approaches to the cervical spine were not used commonly due to the risk of complications, including vertebral artery damage and Horner syndrome [11]. Anterior or posterior approaches have been favored in the treatment of degenerative cervical radiculopathy. Specifically, the anterior cervical discectomy and fusion operation and posterior cervical foraminotomy have been used to alleviate degenerative cervical radiculopathy. The former is favored by many surgeons, but involves challenging decompression of lateral osteophytes, during which there are risks of nerve root and vertebral artery injuries. Additionally, fusion surgery is expensive and may lead to degeneration in adjacent spinal levels.
Posterior foraminotomy was introduced by Frykholm in 1947 and subsequently popularized by Scoville and Whitcomb [12, 13]. It is often used in cases of nerve root compression caused by posterolateral disc herniation or spondylotic foraminal stenosis. Compared with anterior cervical discectomy and fusion, posterior cervical foraminotomy can enable more effective nerve root decompression without vertebral fusion. Nevertheless, it has the disadvantage of requiring substantial neck muscle damage during the creation of a posterior access window, which can leave patients with axial pain and spinal instability [14]. Minimally invasive percutaneous methods for posterior cervical foraminotomy have been developed [6, 15].
In the modern endoscopic technology, P-PECD is employed to achieve decompression sufficient for cervical radiculopathy treatment with outcomes comparable to those obtained with open surgical procedures [6, 15–17]. The outcome of P-PECD was similar to the efficacy results obtained in several retrospective studies with reported therapeutic success rates in the range of 80–97% [15–18]. According to McAnany’s meta-analysis, satisfactory results were obtained in 94.9% of patients treated by way of a minimally invasive approach, with no significant difference between the endoscopic and microsurgical groups [15]. Furthermore, Ruetten et al. demonstrated that PECD was an effective procedure for treating radicular pain caused by paracentral and foraminal disc herniation, which is consistent with our experiences [6]. For patients suffering from osseous foraminal stenosis, Oertel et al. reported that the clinical success rate of PECD was lower than that of patients with lateral disk herniation [18].
Importantly, relative to open procedures, endoscopic surgery has several advantages related to its minimal invasiveness, including reduced intraoperative bleeding, hospital stay duration, and postoperative pain. Indeed, owing to the minimized muscle disruption associated with P-PECD, we observed a low incidence of postoperative axial pain in our patient sample, the majority of whom recovered quickly and could be discharged on the day of surgery. Moreover, we found that the patients’ mean NDI, neck-VAS, and arm-VAS scores were dramatically improved by the 1-week follow-up examination, relative to preoperative baseline values.
Initially, we used general anesthesia for P-PECD at our center, but transitioned to local infiltration anesthesia. Infiltration anesthesia is sufficient to achieve a “painless” operation, while being conducive to intraoperative doctor-patient communication and shortening operating times.
In this series, we found intraoperative O-arm imaging to be convenient, safe, and facilitative of accurate cannula placement, a crucial element in the success of endoscopic surgery. Accurate placement of working channels and the spinal needle is highly important during PECD because spinal needle malposition during PECD may extend operation times, increase morbidity, and increase mortality risk. The O-arm provided both standard two-dimensional (2D) fluoroscopic images and 3D volumetric CT scans. The robotic movements of the O-arm’s gantry allow 2D fluoroscopy image views and multiplanar 3D images to be obtained from any direction easily. In our center, the 2D imaging function of the O-arm system is used for puncture positioning, whereas 3D imaging is used to confirm the position of the working cannula, which can shorten the operation time and reduce radiation exposure for operators. Secondly, the most apparent advantage of the O-arm over the C-arm is the ability to view images in the axial plane. With the anteroposterior and lateral images acquired with a C-arm, it is difficult to assess the trajectories of the spinal needle and working cannula due to interference from the mandible and shoulder joint. Thirdly, O-arm 3D imaging enables the relationship between the working cannula and protruding disc tissue to be determined in the transverse, sagittal, and coronal planes. O-arm-based navigation also could be used successfully in PECD, as demonstrated by Zhang et al.’s study [19]. Although we did not use the navigation instrument function in this study, we benefitted from multiplanar images with direction flexibility, which enabled us to ensure working channel location accuracy and helped to accelerate the steep learning curve of endoscopic surgery [20]. Assisted by O-arm axial images, the surgeon was able to identify the exact relationships among the nerve root, adjacent bone, and offending tissue. Consequently, working channels could be altered as needed to obtain adequate decompression with minimal disturbance of normal tissues.
In addition, the O-arm enabled us to assess the degree of spinal decompression. Inadequate decompression plays an important role in the failure of posterior cervical foraminotomy, which is due, in most cases, to inaccurate assessment of decompression degree [18, 21, 22]. This issue is more prescient in PECD than in open foraminotomy due to limited endoscope mobility and visualization. Traditional 3D fluoroscopy imaging is too ambiguous to enable surgeons to make precise evaluations. The O-arm can provide 3D imaging with resolution nearly as high as in helical CT scans [23]. Intraoperative O-arm 3D imaging can reveal residual stenosis or offending pathogenic tissue, and thus help surgeons to determine when further decompression is needed.
Both P-PECD and anterior PECD (A-PECD) are extensively accepted minimally invasive procedures for degenerative cervical radiculopathy treatment. The surgeon should carefully analyze the herniation site and adjacent structures when selecting the appropriate surgical approach. For the central and paracentral types of cervical disc herniation, A-PECD, which provides direct decompression, should be considered [3]. P-PECD is the preferred method for lateral disc herniation and foraminal stenosis, particularly when the intervertebral space is collapsed (height < 3 mm) or when there are multi-level degenerative pathological changes. The working channel in P-PECD is larger than that in A-PECD, enabling larger bony structures to be removed and providing a broader range of motion that allows two or three segments to be operated on simultaneously [3, 24].
None of the patients in the present study experienced any permanent surgery-related complications. Improper operation of the spinal needle is the main reason for nerve root injury and dural tears, which are difficult to repair when operating through a minimal access exposure approach [25]. Axial O-arm images can help operators avoid these situations. In PECD follow-up, it is important to check for signs of cervical instability and aggravated degeneration. When < 50% of the cervical facet joint tissues are excised posteriorly, stability is not affected [3, 22, 24]. Although the exact percentage of the facet joint tissue removed could not be measured intraoperatively in this study, the surgeon was able to estimate the extent of removal based on intraoperative O-arm imaging. Generally, in this series, the extent of facet joint removal was approximately 30%.
This study had several limitations. Firstly, the follow-up period was relatively short. Extended follow-up studies are needed to assess long-term outcomes with respect to cervical stability. Secondly, the radiation exposure caused by the O-arm system is non-trivial. The surgeon should be aware of this radiation exposure and its implications. In addition to wearing routine radiation protection equipment, radiation-proof material should be used to protect the patient’s reproductive organs. One previous study estimated that the radiation dose associated with O-Arm 3D scanning was about half that of a 64-slice CT scanner [26]. Although the exact radiation dose is not known, we believe the use of radiation minimizing parameters by a skilled technician should make it acceptable [27]. In addition, the radiation dose can be further decreased by limiting the frequency of scanning to time points when high-quality intraoperative images are medically beneficial.
Conclusions
The P-PECD can be used for safe and effective degenerative cervical radiculopathy management. Utilization of an O-arm imaging system during PECD can enable accurate establishment of the working cannula, which facilitates thorough removal of pathogenic tissue and full decompression of the affected nerve.