Introduction
Intensive postoperative pain is one of the most frequent complaints from patients after thoracic surgical procedures. Inadequate treatment of postoperative pain may result in several complications, including atelectasis, pneumonia, respiratory failure, deep vein thrombosis, and intensive chronic post-surgical pain [1, 2]. Video-assisted thoracic surgery (VATS) is minimally invasive and provides better pain control, and is, therefore, becoming increasingly popular [3, 4]. However, even in VATS, postoperative pain is still an important factor, and may affect post-surgical recovery.
In recent years, anesthesiologists and surgeons have become more aggressive with the use of multimodal analgesic plans. Currently, several hospitals have established a customized “Enhanced Recovery After Surgery program” (ERAS), which employs various analgesic programs, including oral analgesic agents, intramuscular morphine injection, an intravenous patient-controlled analgesia (PCA) pump, and continuous epidural analgesia [5]. Nevertheless, large doses of opium-related drugs may result in gastrointestinal dysfunction [6], while epidural analgesia may result in epidural hematoma, dural perforation or hemodynamic instability [7], and nonsteroidal anti-inflammatory drugs (NSAIDs) may result in gastric stress ulcers [8]. The problems mentioned above highlight the need to improve the currently existing postoperative analgesia.
Alternatives to traditional analgesic modalities include ultrasound-guided paravertebral blockade or intercostal nerve blockade, and wound infiltration with local analgesics [9, 10]. In a recent study, thoracic paravertebral block combined with general anesthesia was applied in elderly patients undergoing lobectomy to improve their postoperative cognitive function [11]. However, a single shot of local anesthetic is insufficient because of the relatively short acting time compared with drainage duration [12]; and several complications associated with ultrasound-guided nerve blockade have been reported, including hypotension, vascular puncture, pleural puncture and pneumothorax [13]. Continuous nerve block (CNB) through a thoracoscopic-guided paravertebral catheter (PVC) or intercostal catheter (ICC) could solve some of the problems mentioned above. Several studies on CNB have been published [9, 10, 14, 15], and continuous thoracic epidural analgesia or continuous paravertebral nerve block has been recommended by the Polish consensus statement [16], but reports in which intercostal nerve blockade and paravertebral blockade are compared are limited. Therefore, in this study, we investigated the method of placing a PVC or an ICC in the sub-pleural space followed by continuous injection of ropivacaine by an infusion pump after surgery and determined their efficiency and safety in postoperative pain control.
Aim
The purpose of this study was to present continuous paravertebral nerve block and intercostal nerve block as alternative anesthesia methods and prove their superiority over the traditional anesthesia modalities.
Material and methods
This study was reviewed and approved by the human research ethics committee of Hwa Mei hospital, (No. PJ-NBEY-KY-2019-154-01) and informed consent forms were signed by participants.
Starting in November 2018, we placed PVC during two-port VATS for continuous paravertebral block after surgery. Because of its favorable security and analgesic effect, continuous paravertebral block had been applied as a routine anesthesia method after two-port VATS. Then we hypothesized that postoperative pain was mainly caused because of intraoperative sustained pressure on the intercostal nerve by the thoracoscopic lens and postoperative sustained pressure from the drainage tube. We began placing an ICC into the intercostal space of the drainage tube for continuous intercostal nerve block in April 2019.
In this study, a retrospective database was used to identify patients who underwent two-port VATS at Hwa Mei Hospital from May 2018 to October 2019. In total, there were 269 recorded cases, including various types of operations, such as wedge resection, segmentectomy and lobectomy. All of the patients received two-port VATS, which include a viewing port located at the pivot of the seventh or eighth intercostal space and the midaxillary line and a main access port located at the pivot of the fourth or fifth intercostal space and anterior axillary line, by the same surgical team including an operation surgeon (Jie Li) and an assistant (Xu Song). At the end of the operation, a 26-Fr drainage tube was placed through the viewing port. A total of 59 patients received intercostal nerve block, 64 patients received paravertebral block, and 146 patients received intravenous PCA in combination with NSAIDs. Exclusion criteria were as follows: 1) allergy history to ropivacaine, 2) patients with bleeding tendency or bleeding diseases, 3) patients with mediastinal tumors were not included because the positions of incisions were different, 4) patients who received simultaneous bilateral surgery, 5) the first 10 patients who received PVC or ICC were excluded because of the learning curve effect, 6) patients who had difficulties in using the numerical rating scale (NRS). Preoperative examination of patients included in the study was similar in routine, including enhanced chest computed tomography, craniocerebral enhanced magnetic resonance imaging, blood coagulation function, pulmonary function test, electrocardiography, and abdominal color doppler ultrasound. Age, gender, body mass index (BMI), smoking status, forced expiratory volume in 1 s (FEV1), surgical approach, operative time, blood loss, drainage duration, postoperative hospital day, pain score on postoperative day 0, 1, 2, and 3, as well as discharge day, dosage of tramadol hydrochloride, postoperative complications, and chronic neuralgia 3 months after surgery were collected from the hospital information system.
Patients were divided into different groups depending on anesthesia modalities.
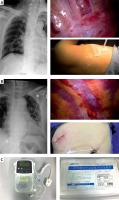
In the CNB group, which was further divided into the PVC group and ICC group, a PVC (Photo 1 A) or an ICC (Photo 1 B) was placed using thoracoscopic guidance during the operation. In the PVC group, the pivot of the seventh intercostal space and subscapular line was chosen for placement of the catheter, and a puncture needle was cautiously inserted into the sub-pleural space without penetrating the parietal pleural tissue. Then 20 ml of ropivacaine (0.25%) was injected so that the sub-pleural space was expanded to include several vertebral levels. Following a guidewire, a 6-Fr, 20 cm-long radio-opaque polyurethane catheter (Specath) (Photo 1 C) was placed over the sympathetic chain to the side of the vertebral body in the sub-pleural space. In the ICC group, a puncture needle was cautiously inserted at the back of the viewing port into the sub-pleural space without penetrating the parietal pleural tissue, and 10 ml of ropivacaine (0.25%) was injected to allow the sub-pleural space to expand. After removal of the needle, a 6-Fr, 20 cm-long radio-opaque polyurethane catheter (Specath) (Photo 1 C) was passed over a guidewire into the intercostal space. Subsequently, 10 ml of ropivacaine (0.25%) was injected at the back of the main access port to the sub-pleural space without inserting a catheter. When the patient returned to the ward, ropivacaine (0.25%) was continuously administered by an electrical infusion pump (Apon) (Photo 1 C) through the PVC or ICC to the sub-pleural space. The infusion pump was set to 5 ml/h and an additional 5 ml infusion was available to the patient, who could manually trigger a single infusion per hour. To check the position of the chest tube and the PVC or ICC, a chest X-ray was obtained at postoperative day 1. If the chest tube and the catheter were in place, the infusion would continue to the day of removal of the chest tube.
Photo 1
A – Placement of PVC by thoracoscopic guidance. The red arrow indicates the PVC. B – Placement of ICC by thoracoscopic guidance. The red arrow indicates the ICC. C – The electrical infusion pump (Apon) and catheter (Specath)
PVC – paravertebral catheter, ICC – intercostal catheter.
In the control group, a 100 ml PCA pump, containing 100 μg of sufentanil and 15 mg of tropisetron, was connected to the central venous catheter. The PCA pump was set to 2 ml/h, and an additional 2 ml infusion was available to the patient for manual administration. In addition, 60 mg of ketorolac tromethamine was injected twice daily at postoperative days 0, 1, and 2.
Patients received health education, including the pain score method with a numerical rating scale (NRS), at admission. A chart card with a 10-cm-long horizontal line with word anchors at each end, ranging from 0 = “no pain” to 10 = “worst pain” was used to assess the postoperative pain. As a demonstration painless ward, the nursing staff was specially trained to use consistent methodology to query patients about pain. The pain score at rest was evaluated three times per day (7 am, 3 pm and 11 pm), and included in our hospital information system.
In all groups, 0.1 g of tramadol was given by intramuscular injection if the patients’ NRS ≥ 4 at rest or NRS ≥ 6 during activity. Since the dosage of tramadol was also an observational index in our study, the NRS of breakthrough pain was not included.
The drainage tube was removed when there was no air leak and the volume of drainage was below 150 ml per day. Then a chest X-ray was taken the day after removal of the surgical drain and the results used to determine the patient’s discharge date. The decision of removal and discharge was made by discussion between the operation surgeon (Jie Li) and the assistant (Xu Song).
Among the 269 patients enrolled, 247 patients were successfully followed up 3 months after surgery. Long-term chronic neuralgia, which included specific incision pain, numbness, pruritus, hyperaphia and occasional muscular spasm, was evaluated and recorded in the outpatient clinic by the operation surgeon (Jie Li) and a member of the nursing staff from the Thoracic Surgery Department.
Statistical analysis
Categorical variables were examined by χ2 test. Continuous data were expressed as the mean ± standard deviation (SD). Before comparison, the continuous variables of two groups were examined by the Levene test. If variances were not equal, the Brown-Forsythe test was performed. In addition, if variances were equal, one-way ANOVA was employed. Calculations were performed using the SPSS statistical package, version 20.0 (SPSS Inc, Chicago, IL – IBM since 2011, USA).
Results
A total of 269 patients met qualifying criteria for enrollment in this study. Demographic data are presented in Table I. The comparison of postoperative outcomes is presented in Table II.
Table I
Demographic and perioperative features of the study patients
Table II
Comparison of perioperative outcomes
In this study, no catheter-related complications or ropivacaine-related acute toxicity was observed. Age, gender, BMI, FEV1, operative time, blood loss, postoperative complications, pain score on postoperative day 1, 2, 3, and discharge day, tramadol hydrochloride requirements, drainage duration, postoperative hospital stay, complications and chronic neuralgia 3 months after surgery between the control group and the CNB group were compared. No significant difference was observed in age, gender, BMI index, FEV1, operative time, or blood loss. However, when compared with the control group, patients in the continuous nerve block group had a lower pain score on postoperative day 0 (p < 0.001), 1 (p < 0.001), 2 (p < 0.001), and 3 (p < 0.001), and on the discharge day (p < 0.001) (Figure 1 A), a lower dosage of postoperative tramadol hydrochloride (p < 0.001) (Figure 1 B), and shorter drainage duration (p = 0.004) and postoperative hospital stay (p < 0.001) (Figure 1 C). In addition, more patients suffered from postoperative pneumonia in the control group (19, 13.0%) than in the CNB group (9, 7.3%), but the difference was not statistically significant (p = 0.092). Furthermore, in the control group, 25 patients had mild to severe gastrointestinal reactions, including nausea, emesis and dizziness, among which in 11 patients, PCA pumps had to be removed prematurely. In the PVC group, only 2 patients had gastrointestinal reactions which might be related to anesthetics used intraoperatively. In the ICC group, there were no complaints about gastrointestinal reactions (Figure 2).
Figure 1
Postoperative pain control. A – Compared with the control group, patients in the PVC group and ICC group obtained a lower pain score on postoperative day 0, 1, 2, 3 and discharge day. There was no significant difference in postoperative day 0, 1, 2, 3 and discharge day between the PVC group and the ICC group. B – Dosage of tramadol after surgery in the control group, the PVC group and the ICC group. C – Drainage duration and postoperative hospital stay in the control group, the PVC group and the ICC group. D – Compared with the control group, more patients in the PVC group and ICC group had no complaints about long-term postoperative pain, and fewer patients in the PVC group and ICC group suffered from moderate and severe pain (NRS ≥ 4)
PVC – paravertebral catheter, ICC – intercostal catheter, NRS – numerical rating scale.
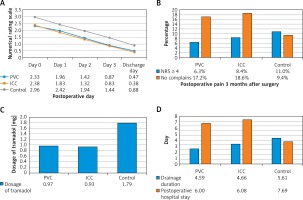
Figure 2
Postoperative complications in the control group, the PVC group and the ICC group
PVC – paravertebral catheter, ICC – intercostal catheter.
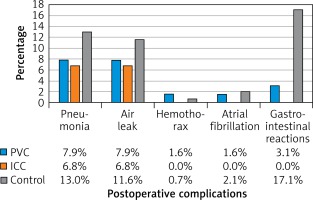
Subsequently, outcomes from the PVC group and ICC group were analyzed. No significant difference was observed in age, gender, BMI index, FEV1, operative time, or blood loss. Furthermore, patients in the ICC group had a similar pain score on postoperative day 0 (p = 0.747), 1 (p = 0.438), 2 (p = 0.575), and 3 (p = 0.755) and on the discharge day (p = 0.353) when compare with patients in the PVC group (Figure 1 A). In addition, no significant difference was observed in dosage of postoperative tramadol hydrochloride (p = 0.874) (Figure 1 B), drainage duration (p = 0.898) or postoperative hospital stay (p = 0.878) (Figure 1 C) between patients in the PVC group and the ICC group. The incidence of postoperative pneumonia was similar in PVC (5, 7.9%) and ICC (4, 6.8%) groups (p = 0.529) (Figure 2).
In this cohort, 26 patients suffered from a prolonged air leak (≥ 3 days), and no one needed a second operation; 28 patients suffered from postoperative pulmonary infection confirmed by chest X-ray; 4 patients suffered from transient atrial fibrillation, which improved after pharmacotherapy; 2 patients suffered from hemothorax, and were successfully treated with conservative therapy; 27 patients suffered from gastrointestinal reactions.
In addition, the long-term chronic pain 3 months after surgery was evaluated and studied (Figure 1 D). Although patients in the control group (16, 11.0%) were more liable to suffer from long-term postoperative pain (NRS ≥ 4) than those in the CNB group (9, 7.3%), the difference was not significant (p = 0.209). Patients in the CNB group (22, 17.9%) were more likely to feel no incision pain than those in the control group (15, 9.4%), but similarly, the difference was not significant. There was also no significant difference between the PVC group and the ICC group.
Discussion
Three-port VATS has been widely used in thoracic surgery and has so far benefited many patients. In recent years, more minimally invasive surgeries, including single-port and two-port VATS, have been developed [17]. However, regardless of the increasing acceptance and number of applications, the benefit of VATS with fewer ports remains controversial as it affects postoperative pain [18]. Nevertheless, fewer ports would mean limited trauma and less intercostal nerve injury. In our hospital, we have developed two-port VATS, single-port VATS, and two-port in single intercostal space VATS. Two-port VATS provide a relatively simple surgical procedure for less experienced surgeons. In this study, patients who underwent surgery performed by the same surgical team were enrolled to reduce sample selection bias. Our surgical team consists of the operator and the assistant – experienced surgeons, who have performed two-port VATS together since 2014. Even though the surgical team had successfully completed hundreds of operations and had years of surgical experience, several patients still suffered from intensive postoperative pain. Therefore, with the help of anesthetists, we endeavored to improve postoperative pain management.
Effective pain control after VATS is critical because of the correlation between postoperative pain management and recovery after surgery. Patients benefit from improved control of postoperative pain, including shorter time to ambulation, reduced pulmonary complications and reduction in the development of chronic pain states [1, 19]. In recent years, regional analgesia techniques have been shown to be effective and safe in postoperative pain control. However, a single shot of local anesthetic is insufficient because of the relatively short effect time compared with drainage duration and liposomal bupivacaine; a sustained release formulation of bupivacaine was used successfully to provide long-acting analgesia after injection of a single dose, but it is very expensive and not widely available [20, 21].
Continuous regional nerve blockade has been successfully used in knee arthroplasty [22], hernia repair [23], and live liver donors [24]. In recent studies, paravertebral blockade and intercostal nerve blockade have been studied after VATS [9, 10]. In most studies, the injection of local anesthetics through ICC tended to be applied in single-port VATS because only one intercostal space can be blocked [25]. In contrast, after injection though the PVC, local anesthetics can spread to several intercostal spaces through the paravertebral space, and intercostal nerves can be blocked at multiple levels [26]. Therefore, PVC was applied in multiple-port VATS. However, studies comparing the efficiency and safety between PVC and ICC in different types of VATS are limited. In theory, PVC may be more advantageous than ICC in the two-port VATS, because ropivacaine can travel to several intercostal spaces through the paravertebral space. However, injection of local anesthetics through PVC might induce self-limited automatic nerve system blockade if local anesthetics result in a high thoracic nerve block [27]. However, we hypothesized that postoperative pain was mainly caused by intraoperative sustained pressure on the intercostal nerve by the thoracoscopic lens and postoperative sustained pressure from the drainage tube. Therefore, we presented an approach that allows for implantation of an ICC in the viewing port to continuously inject ropivacaine, and inject a single dose of ropivacaine for intercostal nerve blockage into the main access port.
In this study, postoperative pain after two-port VATS was analyzed. First, CNB through PVC or ICC was shown to have several advantages over the traditional analgesic strategy in alleviating postoperative pain, shortening drainage duration and postoperative hospital stay, reducing opioid requirements, and increasing tolerance of patients to analgesics. Then, analgesic effects of PVC and ICC were compared, and no significant differences were observed. Due to the rare complications of Harlequin syndrome and hypotension [12, 21] associated with paravertebral block, ICC seems to be the better choice, which might need further research to confirm. Finally, the long-term chronic neuralgia was evaluated. There was no significant difference in long-term chronic pain between the groups. We speculated that there might be two reasons: first, we might need a larger sample size to confirm the difference. Second, there was no specific NRS score for each patient in our follow-up database.
Our study has several limitations. First, this study has a retrospective design, and a relatively modest number of PVC and ICC cases. We will try to present a prospective and randomized study, and might enroll more patients. Second, the follow-up duration is relatively short, and the follow-up data are not sufficient to evaluate long-term chronic neuralgia and catheter-related complications, which would be the focus of our future prospective study.
Conclusions
Continuous ropivacaine infusion through PVC and ICC are both appropriate anesthetic techniques to control postoperative pain in two-port VATS. In cases where blockade of the high thoracic intercostal nerve is required, ICC may be more advantageous than PVC. Because of the limitations mentioned above, a further prospective and randomized study is warranted to verify the conclusion and assess long-term outcomes.









