Introduction
Chronic kidney disease (CKD) is very prevalent, affecting 8–16% of the population globally. Patients with CKD suffer from high mortality and morbidity [1]. Previous studies have indicated that cardiovascular events are the leading cause of death for patients with CKD [2–4]. Current data show that CKD or end-stage renal disease (ESRD) and cardiovascular diseases share some common mechanisms, such as accelerated inflammation and atherosclerosis [5–7].
Pregnancy-associated plasma protein A (PAPP-A) is a newly discovered zinc-binding metalloprotein, which has been suggested to be involved in the processes of inflammation and atherosclerosis [8–10]. PAPP-A primarily acts as a protease cleaving inhibitory binding proteins of insulin-like growth factors [11]. In vitro studies have indicated that proinflammatory cytokine tumor necrosis factor α and interleukin 1β are powerful stimulators in inducing the expression of PAPP-A [8, 10, 12]. In vivo studies have suggested that a mouse model with PAPP-A gene knock-out could resist the accelerated progression of atherosclerosis, whereas over-expression of the gene of PAPP-A in the mouse model could accelerate progression of atherosclerosis [8, 13]. Clinical studies have shown that PAPP-A is not only an acute and sensitive biomarker for the diagnosis of acute coronary syndrome [14–16], but also acts as a prognostic indicator of all-cause mortality and combined cardiovascular events for patients with coronary heart diseases [17–25], which was systematically reviewed in our previous meta-analysis [24].
It remains unclear whether elevated PAPP-A was a predictor in patients with CKD. Some previous studies have shown that PAPP-A is an independent risk factor associated with increased mortality and cardiovascular events, while others have indicated no association in patients with CKD [26–32]. So we combined all available prospective studies and conducted a meta-analysis to assess the association of elevated serum PAPP-A and the risk of all-cause mortality and cardiovascular events in patients with CKD.
Material and methods
Search strategy
We performed this meta-analysis according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [33]. Two authors (Yuehua Li and Xv Meng) identified articles through searching MEDLINE from 2000 to Mar 2017. The key word used in the search was “PAPP-A”. No language restriction was applied for searching and study inclusion.
Study selection and outcomes
The inclusion criteria were determined as follows: (1) prospective studies design; (2) provided the referent (the lowest) and highest levels of serum PAPP-A, or the cut-off value of PAPP-A; (3) provided the unadjusted or multivariable adjusted relative risks (RRs) and their corresponding 95% confidence intervals (CIs).
The primary outcome was all-cause mortality. The second outcome was cardiovascular events, which were defined by each individual study, including cardiovascular death, myocardial infarction, revascularization, sudden death, and so on.
Data extraction
Data extraction was conducted by two independent authors (Yuehua Li and Xv Meng). Discrepancies were resolved by group discussion. The extracted data included the name of the first author, publication year, sample size, number of events, male proportion, mean age, duration of follow-up, assay methods for measuring serum PAPP-A, cut-off value of serum PAPP-A, outcomes, adjusted covariates and RRs and their corresponding 95% CIs. We extracted the most fully adjusted RRs for the highest level of PAPP-A compared with the lowest one. If the multivariable adjusted RRs were not provided, we extracted the unadjusted ones.
Statistic analysis
We considered the hazard ratios or odds ratios as RRs in prospective studies. Compared with the lowest category of PAPP-A, the pooled RRs and their 95% CI of the highest category were estimated by a fixed-effect model to incorporate the inter-study variability. We extracted data from the highest quartile if provided by > 2 quartiles. If there was significant heterogeneity among studies, we used the random-effect model. The heterogeneity was assessed by the Q statistic, I 2 and p-value. I 2 > 50% was considered to indicate significant heterogeneity among the trials [34]. We tried to explore the potential source of heterogeneity by conducting sensitivity analysis and subgroup analysis. We performed the sensitivity analysis by removing each individual study to evaluate the study’s effects on the summary estimate. The subgroup analysis was performed by specified study characteristics, such as age (≥ 65 or < 65 years), male proportion (≥ 60% or < 60%), the follow-up term (≥ 5 or < 5 years), and assay methods (Time Resolved Amplified Cryptate Emission (TRACE) or others).
We assessed publication bias by Begg’s funnel plot and Egger’s regression test [35]. A two-sided p-value < 0.05 was considered to be significant. All of these analyses were completed by STATA software (10.0 version, Stata Corporation, TX, USA).
Results
Search results
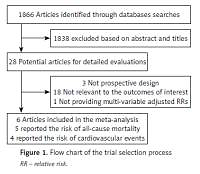
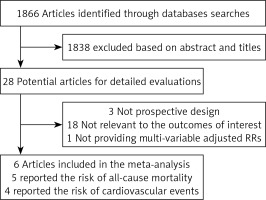
We identified 1866 articles in the initial research. Among these studies, 1838 were excluded based on the title and abstract due to being experimental studies, reviews and non-relevant. Twenty-eight potential studies were selected for detailed accession. We further excluded 22 for the following reasons: not prospective design (n = 3), not relevant to the outcomes (n = 18), did not provide RRs or their 95% CI (n = 1). Finally, 6 potential prospective studies concerning 2034 participants and 792 events were included in our meta-analysis [26, 28–32]. Among them, 5 trials have reported the endpoint of all-cause mortality [26, 28–30, 32], and 4 have reported the endpoint of cardiovascular events [26, 29–31].
Study characteristics
The characteristics of the included studies are presented in Table I. All the studies were conducted in Europe. The sample size ranged from 130 to 1098. The average age range was from 53 to 70 years. The male proportion ranged from 46.7% to 70.9%. Four studies were performed in dialysis patients [28–30, 32], 1 study was performed in patients with diabetic nephropathy [26] and 1 study was performed in patients with ESRD waiting for kidney transplantation [31]. The duration of follow-up were varied from 3 months to 5 years. Serum PAPP-A was measured by TRACE in 3 studies [29, 30, 32], and by other methods in 3 studies [26, 28, 31]. PAPP-A values were obtained by a cut-off value in 4 studies [26, 29, 31, 32], by quartiles in 1 study [30] and the median value in 1 study [28] (Figure 1).
Table I
Characteristics of included studies
| Study | Year | Country | Men% | Mean age | Follow-up term | No. of subjects | No. of death | No. of CV events | Type of CKD |
|---|---|---|---|---|---|---|---|---|---|
| Lauzurica et al. [31] | 2005 | Spain | 65.7 | 53.14 | 49.3 months | 178 | NA | 27 | Renal transplant |
| Astrup et al. [26] | 2007 | Denmark | 70.88 | 61 | 10.1 years | 197 | 48 | 41 | Diabetic nephropathy |
| Etter et al. [32] | 2010 | Swizerland | 65.3 | 70 | 17.5 months | 170 | 23 | NA | Haemodialysis |
| Kalousova et al. [29] | 2012 | Czech Republic | 54 | 63.2 | 5 years | 261 | 146 | 71 | Haemodialysis |
| Kalousova et al. [30] | 2014 | Germany | 55 | 66 | 4 years | 1098 | 534 | 398 | Diabetic haemodialysis |
| Jefferies et al. [28] | 2015 | Finland, UK | 67 | 65.3 | 407 days | 130 | 25 | NA | Haemodialysis |
| Study | PAPP-A assay method | Cut-off value [mIU/l] | Outcomes | Adjusted variables | |||||
| Lauzirica et al. [31] | DSL ELLISA | 1.82 | Cardiovascular event, chronic allograft nephropathy | Age, sex, donor age and sex, type of dialysis, time in dialysis, number of HLA mismatched antigens, cold ischemia, immunosuppressive treatment, acute rejection, chronic allograft nephropathy | |||||
| Astrup et al. [26] | Biotintyramin-amplified enzyme immunoassay | 12.6 | All-cause mortality, cardiovascular mortality | Age, cholesterol, HbA1c, Cr | |||||
| Etter et al. [32] | TRACE | 24 | All-cause mortality | Age, sex, number of comorbidities, dialysis vintage, Kt/V, IL-6, CRP, PTH, Ca2PO4 product, cholesterol | |||||
| Kalousova et al. [29] | TRACE | 30.8 | All-cause mortality, cardiovascular mortality, infectious mortality | Age, sex, hypertension, DM, smoking, BMI, CVD, IGF, MMPS, transplantation, cTnI, BNP, albumin, cholesterol, CRP, orosomucoid, retinol, phosphate, PTH | |||||
| Kalousova et al. [30] | TRACE | 20.9 | All-cause mortality, cardiovascular events , infectious mortality | Age, sex, smoking, BMI, atorvasatin, CVD, SBP, dialysis vintage, cholesterol, Cr, albumin, phosphate, hemoglobin | |||||
| Jefferies et al. [28] | Immunofluorometric assays | 3.45 | All-cause mortality | Age, cTnT, dialysis vintage | |||||
[i] BMI – body mass index, BNP – B type natriuretic peptide, CKD – chronic kidney disease, Cr – creatinine, CRP – C-reactive protein, cTnI – cardiac troponin T, CVD – cardiovascular disease, DM – diabetes mellitus, DSL – Diagnositc Systems Laboratory, F – female, M – male, ELISA – enzyme-linked immunosorbent assay, HLA – human leukocyte antigen, IGF – insulin-like growth factor, IL – interleukine, MMP – matrix metalloproteinase, NA – not available, PAPP-A – pregnancy-associated plasma protein A, PTH – parathyroid hormone, SBP – systolic blood pressure, TnT – troponin T. TRACE – Time Resolved Amplified Cryptate Emission.
Main analysis
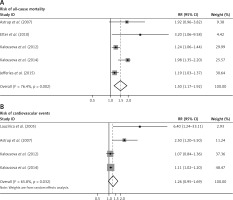
Comparing the group with lowest levels of serum PAPP-A, the pooled RR for all-cause mortality was 1.50 (95% CI: 1.17–1.92, p = 0.001; I 2 = 76.2%, p for heterogeneity = 0.002) (Figure 2 A). For the endpoint of cardiovascular events, the pooled RR was 1.26 (95% CI: 0.95–1.69, p = 0.113; I 2 = 65.8%, p for heterogeneity = 0.032) (Figure 2 B).
Figure 2
Elevated serum PAPP-A and risk of all-cause mortality, cardiovascular events and mortality due to infection. Meta-analysis of the highest vs. lowest serum PAPP-A for (A) risk of all-cause mortality; (B) risk of cardiovascular events
CI – confidence interval, PAPP-A – pregnancy-associated plasma protein A, RR – relative risk.
To clarify the heterogeneity, we performed sensitivity analysis by excluding each individual study at a time (Table II). The sensitivity analysis also showed that the positive relationship between elevated circulation PAPP-A and the risk of all-cause mortality was not modified by excluding each individual study at a time. Furthermore, we conducted subgroup analysis to explore the potential sources of heterogeneity according to the specified characteristics. As shown in Table III, age, male proportion, follow-up term and the assay methods of serum PAPP-A were not modifiable factors to the relevant endpoint of all-cause mortality.
Table II
Sensitivity analysis excluding one study at a time (all-cause mortality)
Table III
Subgroup analysis
Publication bias
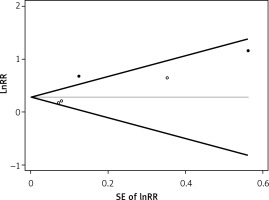
No evidence of publication bias was observed in Begg’s funnel plot. The results was further confirmed in Begg’s adjusted rank correlation test (p = 0.221) and Egger’s regression test (p = 0.176) for the association of elevated serum PAPP-A with all-cause mortality (Figure 3).
Figure 3
Begg’s funnel plot (with pseudo 95% CIs) of the studies in the meta-analysis. Studies that reported the elevated serum PAPP-A and the risk of all-cause mortality were plotted with lnRR on the vertical axis and the SEs of the lnRR along the horizontal axis
PAPP-A – pregnancy-associated plasma protein A, RR – relative risk, SEs – standard errors.
Discussion
To the best of our knowledge, this is the first meta-analysis which has systematically reviewed the association between circulating elevated PAPP-A and the risk of all-cause mortality and cardiovascular events in patients with CKD. In the meta-analysis of prospective studies, we found that elevated serum PAPP-A was associated with 1.50-folder higher risk of all-cause mortality for the patients with renal dysfunction. The relationship of serum PAPP-A and the risk of all-cause mortality was not modified by age, male proportion, follow-up term or the assay methods of serum PAPP-A. There seemed to be a positive association between elevated serum PAPP-A and cardiovascular events, but it was not statistically significant.
Previous studies about the relationship between circulating PAPP-A and prognosis of CKD patients are inconsistent. Some trials have reported a positive association between serum PAPP-A and the risk of death, while others have reported no association [26–32]. We combined all available trials and found a positive association of circulating PAPP-A and the risk of all-cause mortality. Our findings supported the previous meta-analysis which reported that elevated serum PAPP-A was an independent risk factor for all-cause mortality in patients with coronary heart disease [24]. Furthermore, our sensitivity analysis showed that the positive association of the serum PAPP-A and the risk of all-cause mortality was not influenced by excluding each individual study at a time, which strengthened the statistical power. CKD, especially ESRD, are known as a heavy burden with high mortality, morbidity and economic cost. Therefore, it is helpful to identify the biomarkers for risk stratification and prognosis analysis. All of the included trials have controlled numerous factors such as age, gender, serum cholesterol, creatinine and dialysis parameter. We used the most fully adjusted results and found that elevated serum PAPP-A was an independent risk factor for all-cause mortality in patients with CKD.
Heterogeneity is often a concern of a meta-analysis. Although there was significant heterogeneity among the included studies, the sensitivity analysis showed that the main results were not influenced. Furthermore, our subgroup analysis indicated that age, male proportion, follow-up term and assay methods for measuring PAPP-A were not modifiable factors. Nevertheless, large-scale prospective studies are needed to verify the potential factors influencing the association between circulating PAPP-A and all-cause mortality.
It was not fully understood about the elevated serum PAPP-A in predicting adverse events in CKD patients. First, clinical studies have reported that serum PAPP-A is not only correlated with serum level of creatinine, a marker of renal function, but is also correlated with other inflammatory factors, such as C-reactive protein, cytokine tumor necrosis factor α and interleukin 1β in patients with renal disease [31, 36–39]. Second, in vivo studies have indicated that the animal model with PAPP-A gene knock-out was less likely to suffer from nephropathy compared with the wild type in old age [40, 41]. The mouse model without PAPP-A gene expression could resist the development of diabetic nephropathy [42]. Third, in vitro studies have suggested that inflammation cytokine tumor necrosis factor α and interleukin 1β could stimulate PAPP-A gene expression in human mesangial cells, indicating that the kidney may be another target organ of PAPP-A [43]. Two included trials have reported that serum PAPP-A was associated with high risk of infectious mortality in CKD patients, which may be an important cause of death [29, 30]. Fourth, PAPP-A is an important metalloprotein involved in the progression of atherosclerosis [8]. Elevated serum PAPP-A was correlated with the severity of the plaque load [44]. Accelerated atherosclerosis is an important cause of the adverse prognosis in patients with CKD [2, 4, 45, 46]. Our meta-analysis indicated that circulating PAPP-A seemed to be associated with the risk of cardiac events in the CKD patients, but it was not statistically significant. Restricted by the number of included trials and heterogeneity, it needs further exploration in experimental studies and large-scale prospective studies for PAPP-A in predicting cardiovascular events in CKD patients in future.
Our study has a few limitations. First, the residual confounders may be potential confounders. Most included studies have adjusted for a wide range of potential confounders. However, we could not exclude other confounders which might have influenced the results. For example, serum PAPP-A level might be affected by heparin administration. Three included trials [28–30] have indicated that blood samples were collected before heparin administration while others did not. So it needs further evaluation whether heparin administration is a modifiable factor for the predictive value of serum of PAPP-A. Previous history of coronary artery disease may also be a modifiable factor for the prognosis. We could not perform a subgroup analysis according to the percentage of previous history of coronary artery disease because only three studies have provided the relative data [26, 29, 30]. Second, restricted by the number of included trials, the predictive value of serum PAPP-A in cardiovascular events and all-cause mortality needs further exploration in future studies. Third, the results may be influenced by study design, but we did not find that the predictive value of PAPP-A was influenced by age, male proportion, duration of follow-up or assay methods. Fourth, all of the included studies had different cut-off values of serum PAPP-A. Given the nature of a meta-analysis, we could not provide the cut-off value of serum PAPP-A. Fifth, necrosis factor α and interleukin 1β are stimulators of PAPP-A. However, we could not compare serum PAPP-A with tumor necrosis factor α and interleukin 1β about the predictive value because only two included trials have provided the relative data about tumor necrosis factor α and interleukin 1β. Finally, publication bias could be a concern, but we found no evidence of publication bias.
In conclusion, elevated serum PAPP-A was associated with higher risk of all-cause mortality in patients with CKD. The independent predictive value of PAPP-A was not affected by age, male proportion, duration of follow-up term or assay methods. How PAPP-A was involved in kidney disease and whether serum PAPP-A is a candidate biomarker for prognosis of renal dysfunction needs further exploration in numerous experimental and large-scale clinical trials.