Introduction
Presacral tumors are a rare group of heterogeneous lesions located in a potential space referred to as the retrorectal or presacral space. The estimated incidence of lesions in this region is 1/40,000 admissions [1, 2]. The classification of presacral tumors takes into account histology (benign or malignant tumors) and origin (congenital or acquired).
The presacral location is responsible for atypical symptoms with varying nature and intensity depending on the tumor size and coexisting inflammation of surrounding tissues. The occurring symptoms are directly related to compression or infiltration of anatomical structures of the pelvis minor by the tumor and may manifest as lower back pain or rectal pain or resemble various neurological defects. However, often presacral tumor are asymptomatic; completely asymptomatic lesions occur in 26–50% of patients [3, 4]. Lack of characteristic symptomatology and difficult anatomical localization make the diagnostic process difficult and often delays the ultimate diagnosis [3, 4].
The cornerstone of diagnosis of presacral tumors is the digital rectal examination (DRE), with sensitivity approaching 97% [5]. Transrectal ultrasound imaging (TRUS), computed tomography (CT) and magnetic resonance imaging (MRI) provide assessment of their topography and relation to the other anatomical structures of the pelvis minor [1, 5, 6].
Irrespective of histologic grade, the treatment of choice for presacral tumors involves surgical intervention from either an abdominal, transsacral, or mixed approach with or without resection of the cocygeal bone. Selection of the surgical technique depends on the size and level of the tumor location with respect to the S3 vertebra. Some histological types of presacral tumors require additional chemo- or radiotherapy, but such management is controversial due to high radioresistance of tumors of this region [3].
The aim of this study was to analyze cases of presacral tumors that underwent surgical treatment at the Department of General and Colorectal Surgery, Medical University of Lodz between 2003 and 2012 with regard to diagnostics, methods and outcomes of treatment.
Material and methods
The study enrolled patients who underwent surgical treatment due to presacral tumors at the Department of General and Colorectal Surgery, Medical University of Lodz from 2003 to 2012. The study data were retrospectively collected from medical records, surgical protocols, histopathology reports and a questionnaire completed by patients. The data were analyzed for age, gender, clinical symptoms, type of conducted diagnostics procedures, histopathology results, type of implemented treatment, intra- and perioperative complications as well as early and long-term treatment outcomes. Follow-up was evaluated based on data from outpatient clinics of surgery and a questionnaire completed by all patients who had undergone surgical treatment. In all patients the surgical approach for presacral tumors is established by appropriate imaging methods (TRUS, CT and MRI), which demonstrate the location, nature, and size of the lesion as well as the involvement of adjacent viscera, sacrum, or pelvic sidewalls. The extent of surgery is determined by the character of the tumor, and in all cases the decision was made intraoperatively.
Results
The study enrolled 29 patients who underwent surgical treatment for presacral tumors. This group included 16 men (average age: 48 years) and 13 women (average age: 46 years). Surgical treatment for presacral tumors accounted for 0.19% of all surgical procedures performed in the analyzed period at the Department of General and Colorectal Surgery, Medical University of Lodz.
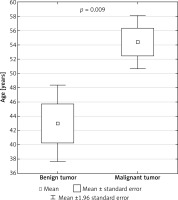
Malignant tumors accounted for 34% of all surgical cases (n = 10), and 80% of them occurred in men. Benign tumors accounted for 66% of cases (n = 19) and they occurred predominantly in women (58%). Benign lesions were more common in women, while malignant lesions were more common in men (p < 0.05). The average age of patients with benign tumors was lower than that of patients with malignant tumors (43.0 vs. 54.4 years) (p < 0.05) (Figure 1).
There were 8 metastatic lesions among malignant tumors, from: large intestine (n = 5), kidney (n = 2) and prostate (n = 1) and 2 primary lesions – teratocarcinoma (n = 1) and carcinoid (n = 1). The benign tumors included: cysts (n = 5), fibromas (n = 3), teratoma (n = 2), hamartoma (n = 1), neurogenic tumor (n = 1), lipoma (n = 1) and tumors of inflammatory etiology (n = 6).
Thirty-eight percent of cases were asymptomatic. Statistical analysis did not reveal a correlation between presence of complaints related to a presacral tumor and histology of the tumor. Chronic pain of the sacral region was the most common symptom (n = 13, 45%). Other reported symptoms included constipation (n = 5, 17%), fever (n = 4, 14%), vomiting (n = 4, 14%), pain upon defecation (n = 3, 10%) and loss of body weight (n = 3, 10%) and benign lesions with respect to presenting symptoms (Table I).
Table I
Symptoms reported in patients with presacral tumors
Statistical analysis did not reveal a correlation between histology of the presacral tumor and average time from the first symptoms to initiation of surgical treatment (11 months).
All tumors were evaluated with preoperative imaging studies. Tumors in the presacral space were evaluated based on TRUS imaging in 83% of patients (n = 24), CT in 66% of patients (n = 19) and MRI in 17% of patients (n = 5). Seventeen percent of patients (n = 5) required TRUS, CT and MRI to provide detailed assessment of an advanced lesion.
Imaging studies determined the location of the tumors in relation to the S3 vertebra to enable selection of the surgical approach. In 52% of cases (n = 15), the upper margin of the tumor extended beyond the S3 vertebra, and in 48% of cases (n = 14) the whole tumor was below this level.
A transsacral approach was used in 51% of patients (n = 15), abdominal laparotomy in 41% of patients (n = 12) and a mixed approach in 7% (n = 2). The coccygeal bone was resected along with the tumor in 10% of patients (n = 3) due to a high degree of tumor progression. Partial rectal resection was required in 21% of cases (n = 6) due to malignant infiltration in 5 patients and due to intraoperative injury of the rectum in 1 case.
Cure was achieved in 72% of patients (n = 21), including in 67% after a first surgical procedure (n = 14). Thirty-three percent of patients (n = 7) required reoperation due to impaired drainage and development of hematoma (n = 4), recurrent malignancy (n = 2) and incomplete resection of the lesion (n = 1). In 28% of patients (n = 8) the tumor was assessed as unresectable during the procedure due to extensive infiltration of the surrounding structures of the pelvis minor that was not demonstrated by imaging studies.
Early postoperative complications up to 30 days after the procedure were found in 21% of patients (n = 6): bleeding (n = 2), wound infection (n = 2), urethral injury (n = 1) and injury of the rectal wall (n = 1). Long-term complications were reported in 34% of cases (n = 10), and low back pain was the most common of them (n = 4). No correlation was found between the histology and incidence and nature of early complications.
The average patient follow-up was 4 years, and 7% of patients died during this period (n = 2). Both these deaths were caused by a malignant presacral tumor – 1 case of teratocarcinoma and 1 case of a metastatic lesion. In the teratocarcinoma case, the surgical resection of the primary lesion was a palliative procedure, while in the case of the metastatic lesion, the malignant process became metastatic, resulting in death of the patient.
Local recurrence was found in 11% of patients with a benign lesion and in 40% of patients with a malignant lesion. The neoplastic process recurred more often in malignant than benign cases (p < 0.05). Recurrences of benign tumors were caused by lack of complete resection.
In 7% of patients (n = 2) gas and stool incontinence occurred after resection of a presacral tumor. In both these cases the tumors were malignant. However, no significant correlation between histology or surgical approach and anorectal disorders was found.
Discussion
The presacral region is a potential space located in the pelvis minor. The rectum is its anterior wall, the presacral fascia covering the sacral bone and coccygeal bone is its posterior wall, the peritoneal recess is its superior border, while the lavatory ani muscle is its inferior wall; its lateral borders are delineated by the iliac vessels and ureters. This space includes multiple branches of the sacral plexus, the pelvic visceral nerves, hypogastric nerves and autonomic innervations from the superior and inferior hypogastric plexus. Iliosacral, middle rectal, medium sacral vessels and plexuses of the presacral vein create a rich vascular network here. The whole presacral region is filled by reticular tissue and adipose tissue along with a network of lymphatic vessels. The rich vascular supply and presence of lymph nodes favor location of malignant metastases there. During embryogenesis, this space includes the notochord, large intestine, and posterior intestine, which involutes with time. Due to such diverse embryogenesis, the presacral region can be a site of variable neoplastic lesions, originating from all three embryonic germ layers [1, 5, 7].
Presacral tumors are a heterogeneous group of lesions with diverse histology. The literature classifies them into benign or malignant and congenital or acquired lesions. Further subclassification is based on the tumor histology [8]. According to the literature, the most common presacral tumor is the congenital benign teratoma. However, our study did not support this – as many as 93.1% of lesions were acquired and only 2 tumors were congenital, and they were indeed teratomas. This discrepancy may result from a small sample size and age range excluding patients below 18 years of age, in whom congenital lesions may have already been diagnosed and radically cured at earlier ages [8].
With regard to histology, benign tumors predominated in our study (65.5%), including mainly inflammatory lesions and cysts. Metastases accounted for most of the malignant tumors; again according to literature reports, chordoma is the most common tumor in this setting. In our study sample, benign tumors predominated in women. Malignant tumors accounted for 34.5% of presacral tumors and predominated in men (80%) (Table II) [1, 9–15]. These results are supported by literature data indicating that benign tumors are more common in women, malignant ones in men. Benign lesions are more common in younger subjects. This was also true in our study, where the average age of patients with a benign tumor was 43 years, and with a malignant one 54.4 years. Comparing these results with those of other authors may be biased because many researchers exclude from their study groups the pediatric population [9–15].
Table II
Detailed classification of tumors observed in our study and comparison to literature data
Presacral tumors are usually diagnosed late due to a lack of specific clinical signs and symptoms or their asymptomatic nature. Approximately 26–50% of patients, in the literature, are asymptotic, which is mirrored by our study (38% of patients did not experience any clinical symptoms). Pain accompanying presacral tumors is dull, poorly localized and radiating. Presence of symptoms is believed to indicate complications of presacral tumors, resulting from infiltration of adjacent nerves or vessels. Infiltration of the sacral plexus causes pain of the lower extremities and buttocks. Presacral tumors may be one of the causes of lower back pain. Patients often complain of piercing sacral or perineal pain and gas or stool incontinence [1, 4, 7, 14]. Pain in the sacral or perineal region may also be caused by ongoing inflammation in the pelvis minor. Uncharacteristic chronic sacro-perineal pain was the most common symptom in our study (occurring in 45% of patients). Various degrees of urine, stool and gas incontinence may result from infiltration or mechanical compression of pelvic nerves; stool incontinence was observed in 3% of the study population. Expanding presacral tumors may cause mechanical obstruction of the pelvic outlet and obstruct defecation. Seventeen percent of patients in the study group experienced constipation, 10% reported painful defecation and 3% reported pencil thin stools. The uncharacteristic clinical presentation of lesions in this region may result in establishment of incorrect diagnosis: pilonidal sinus, fistula or perianal abscess. Therefore, diagnostic workup of presacral tumors should be based on a differential diagnosis that includes the above-mentioned disorders [1, 9–15].
Presacral tumors are usually accidental findings of digital rectal examination or vaginal examination conducted for unrelated reasons. This is due to the fact that they are indolent and asymptomatic. Digital rectal examination is conducted in all patients, and our study indicates that its sensitivity approaches 62% [14]. Due to the lack of characteristic signs and symptoms, diagnosis of a presacral tumor may be based on a detailed physical examination and interview, including detailed neurological examination. Rectoscopy to exclude colorectal pathologies should be the first additional test to be performed [14, 16].
Detailed imaging of the tumor and adjacent tissues should be an important part of the diagnostic management of presacral tumors. The CT and MRI seem to be the gold standard, and the best outcomes are provided by a combination of these two modalities. CT best visualizes the skeletal parts, the nature of the tumor – solid or cystic – and infiltration of adjacent anatomical structures. On the other hand, MRI visualizes soft tissues and detailed spatial relations between the structures of the pelvis minor, which is very important for planning a surgical procedure [8, 14, 17, 18].
Preoperative biopsy is of utmost importance for determination of further therapeutic management, as with any tumor [2]. In presacral tumors such a procedure is particularly problematic due to difficult access to the lesion. However, the results of this investigation determine further therapeutic management. It is especially important in Ewing’s sarcomas and chordomas, since in such cases neoadjuvant chemotherapy should be initiated. Based on the experience of various centers, Dozois et al. prepared recommendations on preoperative biopsy [2]. Biopsy should be performed by a radiologist, using a transperineal or transsacral approach with concurrent resection of the lymph node package. Before the procedure each patient should undergo blood clotting tests to reduce the risk of bleeding and biopsy specimen contamination.
Neoadjuvant chemotherapy is an important therapeutic component for selected types of presacral tumors such as Ewing’s sarcoma, osteosarcoma or chordoma. An imatinib (tyrosine kinase inhibitor) treatment cycle is recommended in the therapy of these tumors. Transcatheter arterial embolization (TAE) is recommended for chordomas to increase the effectiveness of resection [19].
The role of radiotherapy in the treatment of presacral tumors is not clearly documented and is not associated with a good therapeutic effect. Carbon ion radiotherapy (CIRT) can be used in the treatment of chordomas. Positron emission tomography (PET) is usually performed to monitor therapeutic effectiveness in patients. Preoperative treatment is used to reduce the size of the tumor and improve the 5-year survival rate [2].
After detailed diagnostic workup and possible neoadjuvant therapy, each patient with a presacral tumor is prepared for the surgical procedure [19]. A surgical team should be provided with a complete set of imaging studies to determine the appropriate approach and resection margin. Preferably in all patients nutritional status should be assessed before surgery and supplemented with total parenteral nutrition (TPN) or enteral nutrition when indicated. Insertion of an inferior vena cava filter is recommended to reduce cardiovascular complications during the procedure. Patients who underwent radiotherapy should undergo temporary catheterization. In presacral tumors a team of anesthesiologists should be prepared for possible intraoperative blood transfusion due to the high risk of intraoperative bleeding. Each patient should receive a prophylactic dose of an antibiotic 48 h before the procedure, and an enema should be used to cleanse the bowels [13].
Selection of the surgical procedure depends on multiple factors. The following factors should be considered in the qualification process: tumor size, its precise location in relation to adjacent anatomical structures, degree of infiltration of adjacent tissues and degree of vascularization. Special attention should be paid to its relation to the S3 vertebra, location relative to the rectum and adjacent vascular plexuses and nerves [13, 20–24].
Currently several methods of surgical approach to the presacral region are known and used. They include transsacral, abdominal, mixed abdomino-sacral, transrectal (transsphincter) and transvaginal approaches. Despite the fact that presacral tumors belong to the area of open surgery, there are also reports documenting laparoscopic techniques used for such treatment [13, 20–24]. Transsacral, abdominal, and mixed abdomino-sacral procedures are most common. Small lesions that do not exceed 1 cm, located below the third sacral vertebra (S3), allow for transsacral resection. Lesions with a diameter larger than 1 cm or located above S3 undergo resection using an abdominal or mixed approach.
Each procedure requires general anesthesia [20–24].
A transsacral or posterior approach involves accessing the presacral space through a vertical incision, approximately 8–10 cm in length, at the S3 vertebral level. Sometimes the sacral or coccygeal bone needs to be resected to enable radical resection [20–24]. Intraoperative assessment of the tumor location and degree of involvement of adjacent tissues in the presacral space are important aspects of this procedure. Special caution in tumor dissection is advised due to the rich and variable blood supply of this region. The advantage of the procedure with a transsacral approach is shorter postoperative recovery. The transsacral approach, without the abdominal approach, is the most commonly selected method [20–24]. This is also supported by our study: 51% of patients underwent a transsacral procedure.
The abdominal, anterior approach (laparotomy) is used for the treatment of larger tumors, with the margin located above the S3 vertebra level. Resection from the abdominal approach involves accessing the presacral space through a midline incision in the lithotomy position. This procedure requires careful dissection of vessels and the mesorectal space. Damage of sacral artery branches may result in intraoperative bleeding that is difficult to manage [25–27]. Abdominal resection is commonly used, which is supported by our study: this was the method of choice in 41% of patients diagnosed with a presacral tumor.
A mixed, abdomino-sacral approach, combining both the above-mentioned techniques, is also commonly used. It is preferred for the treatment of large tumors located above the S3 vertebra, with marked involvement of surrounding tissues. Seven percent of our patients underwent this procedure [25–27].
Very small and low-lying tumors may be resected using a transsphincter or transvaginal approach; however, these methods are used uncommonly [20, 22, 25–27].
Irrespective of selection of the method, surgical treatment aims at complete resection of the tumor with a large margin of healthy tissues to obtain the best possible treatment outcomes. Complete tumor resection increases the chance of cure and reduces the recurrence rate [25–27].
Postoperative radiotherapy is not a standard management of presacral tumors. Treatment of some malignant tumors may be supplemented by a radiotherapy cycle, but its efficacy has not been definitely proven [28].
If the presacral tumor is a cyst or an abscess, the management of choice is to remove it after its drainage using a percutaneous drainage set [25].
Irrespective of selection of the surgical approach, the procedure is associated with high intra- and perioperative risk of complications. The most common complications include hemorrhage, rectal injury, injury of sacral plexus nerves or injury of the urethra [28–31]. Complications of a similar nature were found in 26% of patients in our study. Bleeding, wound infection, and injury of the urethra or rectum were the most common. Bleeding from presacral venous plexuses is the most common cause of intraoperative deaths in patients undergoing surgical treatment for a tumor located in this space. According to the literature, preoperative embolization of blood vessels supplying the tumor and intraoperative pressure control reduce the bleeding risk [25, 28–31]. Lack of tumor recurrence is an important aspect of effectiveness of a surgical procedure. Appropriate oncological cleanness associated with resection of the tumor with an adequate margin of healthy tissue minimizes the risk of local recurrence. Neoplasm recurrence occurs more often with malignant tumors. Chondroma has the highest recurrence rate [29]. According to various studies, presacral tumors recur in 0–15% of cases [20–31]. If a benign tumor is completely resected, we can assume that the local recurrence rate is 0%. Such outcomes are supported by large studies performed by Glasgow et al., demonstrating that the recurrence rate in 22-month follow-up was 0% [14]. In our study the local recurrence rate was 11% for patients with benign tumors and 40% with malignant tumors. Recurrences were caused by lack of complete tumor resection in cases of benign tumors.
Patient survival is an important aspect of effectiveness of therapy. According to studies conducted by Mayo Clinic, 5-year survival after the surgical therapy is 75% [1]. Cody, Macrove, and Quan reported 69% 5-year survival and 50% 10-year survival rates in patients who had undergone surgical treatment for presacral tumors [10, 32]. Spanish studies conducted in Valencia documented a 90% survival rate over 3.5 years of follow-up [29]. All the above figures refer to malignant tumors. In benign tumors, complete tumor resection does not affect the patient survival. Two patients died during 4 years of follow-up in our study. In the first case of teratocarcinoma, resection of the primary lesions was a palliative procedure, while in the second case, after resection of a metastatic lesion the malignancy became metastatic, resulting in the patient’s death.
In conclusion, presacral tumors are a rare condition with an unspecific clinical presentation. Lesions with such location are associated with diagnostic difficulties and are often diagnosed late. Assessment of tumors with imaging studies is a key component for selection of the technique of surgical resection that is the principal therapy for these lesions. Presacral tumors are more common in men and more commonly are malignant tumors in this group. Benign tumors are more common in women. The success rate of surgical treatment is 72% and selection of the surgical approach does not affect the final treatment outcome. Recurrences are more common in malignant tumors.