Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disease [1]. Studies have shown that AD accounts for more than half of all patients whose main clinical symptoms are progressive cognitive decline [2, 3]. The pathology of AD is defined as extracellular aged pigment deposition and intracellular tangled neurofibrils and selective loss of synapses and neurons [4]. A common characteristic of AD is amyloid-β (Aβ) accumulation [5]. Amyloid-β, a 39–43 amino acid peptide, is produced by amyloid precursor protein cleavage [6]. Between the two Aβs, Aβ25–35 exerts neurotoxic effects such as learning and memory impairment, neuronal apoptosis, and oxidative stress. Therefore, Aβ25–35 is used to establish AD models for studying the neurotoxic properties of Aβ and for drug screening [7].
Studies have shown that the generation of AD is closely related to cell apoptosis [8–10]. Cell apoptosis occurs in primary neurons and neuronal cells when exposed to Aβ [6, 11]. Researchers found that a large number of apoptotic neurons existed in the brains of AD patients and that programmed cell death played an important role in central nervous development and the adult central nervous system [12]. Regarding cell apoptosis, previous studies demonstrated that the mitogen-activated protein kinase (MAPK) signalling pathway was of vital importance [13, 14]. MAPK is an intracellular serine/threonine protein kinase, which mainly consists of extracellular signal-regulated kinase 1/2 (ERK1/2), Jun N-terminal kinase (JNK) and p38-MAPK signal transduction pathways [14]. The MAPK/ERK cascade plays a very key role in cell growth, development, differentiation and apoptosis [15]. Runden et al. believed that brain injury activates ERK1/2 and increases p-ERK1/2 to mediate the death of neurons [16].
The occurrence of AD is associated with sex, and hormone replacement therapy reduces the incidence of AD for perimenopausal and postmenopausal women [17–19]. However, the long-term use of oestrogen increases breast cancer, endometrial cancer, stroke, and the risk of heart disease and other diseases [20]. Phytoestrogens have been reported as a safe choice to replace oestrogen, which could counter the toxic effect of Aβ25-35 and protect nerve cells treated with Aβ [21–23]. Kaempferol, a type of phytoestrogen, is a flavonoid that can prevent cancer, antagonize inflammation and microorganisms, and produce a certain protective effect on neurons [24–26]. Previous studies have confirmed that kaempferol could be used as a possible therapeutic agent against the progression of AD [27, 28]. However, the molecular mechanism involved is unclear, and further studies are needed to clarify this issue.
In this study, we explored the underlying molecular mechanisms of kaempferol against the progression of AD. The results showed that kaempferol protected pheochromocytoma (PC-12) cells from Aβ25–35-induced cell death, promoted the expression of anti-apoptotic molecules and inhibited the expression of pro-apoptotic molecules. Our results suggest that the inhibition of apoptosis by kaempferol is mediated, at least in part, through the ER/ERK/MAPK signalling pathway.
Material and methods
Cell culture and experimental design
PC-12 cells were purchased from the cell bank of the Chinese Academy of Sciences and cultured in DMEM complete medium under standard conditions (37°C, 21% O2 and 5% CO2). DMEM basic culture medium, foetal bovine serum and dual antibodies were evenly mixed at a ratio of 89 : 10 : 1, and then DMEM complete culture medium was prepared and stored at 4°C for later use. After adding the appropriate complete culture solution, the resuscitated cells were inoculated in culture bottles at 37°C and 5% CO2. The solution was changed every 24 h. When the cells grew to approximately 80% confluence, 0.25% (w/v) trypsin solution was used to digest the cells for passage.
The PC-12 cells at the logarithmic growth stage were cultured in culture bottles and placed in incubators at 37°C and 5% CO2. After 24 h, the cells were divided into the following groups: the blank group was cultured in DMEM only; the control group was cultured in DMEM with DMSO; the Aβ25-35 group was cultured in DMEM for 4 h, and Aβ25-35 (20 μM) was added for another 24 h; the oestradiol group was cultured in different concentrations (10–2, 10–3, 10–4, 10–5, and 10–6 μM) of oestradiol for 4 h, and Aβ25-35 (20 μM) was added for another 24 h; the kaempferol group was cultured in different concentrations (10–2, 10–3, 10–4, 10–5, and 10–6 μM) of kaempferol for 4 h, and Aβ25-35 (20 μM) was added for another 24 h; the kaempferol + fulvestrant group was pretreated with 1 μM fulvestrant for 1 h, cultured in 10–3 μM kaempferol for 4 h, and Aβ25-35 (20 μM) was added for another 24 h; and the kaempferol + U0126 group was pretreated with 10 μM U0126 culture for 20 min, cultured in 10–3 μM kaempferol for 4 h, and Aβ25-35 (20 μM) was added for another 24 h. The cell vitality, apoptosis rate and the expression of proteins and genes were detected under the same experimental conditions.
MTT assay
The MTT assay measures cell proliferation rate and cell viability by chromogenic changes in MTT dye. The cells were seeded in a flat-bottomed 96-well microplate in quintuplicate, and 20 μl of MTT (5 mg/ml) was added to each well. After incubation for 4 h at 37°C, 200 μl of DMSO was added. The absorbance at 570 nm was determined using a microplate reader (MK3, Thermoelectric, China).
Detection of apoptosis by flow cytometric analysis
Cell apoptosis was quantitatively detected by flow cytometry using an HPC-150 (HPC-150, Handyem, Canada) apoptosis detection kit (BD Pharmingen, USA). Briefly, PC-12 cells were collected by centrifugation (1000 rpm, 15 min) after trypsinization with trypsin (SH30042.01, HyClone, USA). Subsequently, the cells were resuspended in phosphate buffered solution (PBS) and counted. 4–12 × 104 cells were added per group and stained with 195 µl of Annexin V-FITC solution according to the manufacturer’s instructions. Then, 5 µl of Annexin V-FITC and 10 µl of PI were added. Finally, the PC-12 cells were analysed for the apoptotic cell ratio.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to detect the mRNA expression levels of Bcl-2, Bax and caspase-3. Total RNA was extracted using TRIzol reagent (Invitrogen, USA) and then reverse-transcribed into cDNA using an AMV first strand cDNA synthesis kit (B532445, Sangon, China) according to the manufacturer’s instructions. The concentration of RNA was measured using a Nano-100 micro spectrophotometer. The ratio of nucleic acids at 260 nm and 280 nm was used to evaluate the purity of the RNA (nucleic acid has strong absorption near 260 nm and protein has strong absorption near 280 nm). The closer the ratio of nucleic acids at 260 nm and 280 nm is to 2.0, the higher the RNA purity. The RNA obtained in this experiment had a high concentration and purity, as shown in Table I. Primers designed by Primer Premier Version 5.0 software are shown in Table II. The amplification and melting curves of Bcl-2, Bax, Caspase-3 and β-actin are also shown (Figure 1). To investigate the expression of mRNA, cDNA was amplified using a SGExcel UltraSYBR Mixture (B532956, Sangon, China) in a standard volume of 20 μl, which included 10 μl of master mix, 0.4 μl of forward primer, 0.4 μl of reverse primer, 0.8 μl of cDNA, and 8.4 μl of nuclease-free water. PCR amplification was performed using the following protocol: 95°C for 3 min and then 40 cycles of 95°C for 20 s, and 60°C for 30 s. Standard curves were run in each assay, which produced a linear plot of the cycle threshold (Ct) against log (dilution). The expression levels of the target gene were quantified based on the concentration of the standard curve and are presented as relative Ct values. The transcription level of β-actin served as a loading control.
Table I
Quality and quantity of RNA
| Groups | Mass concentration [mg/l] | Absorbance (OD) |
|---|---|---|
| Blank | 160.372 ±6.619 | 1.8995 ±0.0487 |
| Aβ25-35 | 156.779 ±9.276* | 1.8991 ±0.0275** |
| Kaempferol | 155.915 ±3.242*# | 1.8979 ±0.0219**## |
| Oestradiol | 178.660 ±11.353*# | 1.8966 ±0.0323**## |
| Kaempferol + fulvestrant | 183.216 ±7.628*#& | 1.8995 ±0.0437**##&& |
| Kaempferol + U0126 | 173.273 ±8.536*#& | 1.8991 ±0.0298**##&& |
Table II
Primers used for real-time PCR
Figure 1
Amplification and melting curves of Bcl-2, Bax, caspase-3 and β-actin. A – amplification curve of Bcl-2, B – melting curve of Bcl-2, C – amplification curve of Bax, D – melting curve of Bax, E – amplification curve of caspase-3, F – melting curve of caspase-3, G – amplification curve of β-actin, H – melting curve of β-actin

Western blot analysis
Protein levels were detected by Western blotting, with β-actin as an internal reference protein. PC-12 cells were homogenized using RIPA lysis buffer (Invitrogen, USA). The protein concentration was quantified with a BCA protein kit (P0012S, Beyotime), and the absorbance values were measured at 562 nm. Equal amounts of protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (MAB1532, Merck Millipore, Billerica, MA, USA). Subsequently, the transferred PVDF membranes were blocked with 5% non-fat milk in TBST for 1 h at room temperature and then incubated with the appropriate concentration of primary antibodies (Bcl-2, PR-0257, Bax, PR-0258, Caspase-3, ZS-71408, Zsbio Commerce Store, China; ERβ, bs-0116R, p-ERK1/2, bs-3292R, BOSTER, Wuhan, China) at 4°C overnight. On the second day, after washing three times in TBST, the PVDF membranes were incubated with HRP-conjugated goat anti-rabbit IgG (H+L) secondary antibodies (BA1054, BOSTER, Wuhan, China) for 2 h at 37°C. The immunoreactive bands were visualized with an enhanced chemiluminescence (ECL) kit (P0018S, Beyotime, Shanghai, China), and grey values were analysed using a Lane ID gel analysis system.
Statistical analysis
Statistical analyses were completed using SPSS 18.0 software. All data are presented as the mean ± SD (standard deviation) from the results of at least three independent experiments. Statistical analysis among multiple groups was analysed by one-way analysis of variance (ANOVA), and comparisons among groups were performed using Tukey’s test. A value of p < 0.05 was considered statistically significant.
Results
Kaempferol rescued PC-12 cell viability
The cytotoxicity of Aβ25–35 at different concentrations (0.1, 1, 10, and 20 μM) was examined by MTT assay. According to the results, the proliferation rate of the control group had no clear change, and the Aβ25–35 group had significantly reduced viability in a dose-dependent manner compared with that of the blank group (p < 0.01). Then, 20 μM Aβ25–35 was selected as the final concentration because of its apparent effect on cell damage. Compared with the proliferation rate in the Aβ25–35 group, the cell proliferation rate in the oestradiol group was significantly higher (p < 0.01) at different concentrations (10–2, 10–3, 10–4, 10–5, and 10–6 μM). Treatment with 10–3 μM oestradiol induced a clear protective effect on PC-12 cells against damage by Aβ25–35, and so this concentration was used for subsequent studies (Table III).
Table III
Effects of oestradiol on activity of PC-12 cells damaged by Aβ25-35 (± S, n = 6)
| Groups | Concentration [μmol/l] | Absorbance (OD) | Cell proliferation rate (%) |
|---|---|---|---|
| Blank | – | 0.609 ±0.003 | 100 |
| Control | – | 0.609 ±0.005** | 100** |
| Aβ25-35 | 20 | 0.448 ±0.009** | 74** |
| Oestradiol + Aβ25-35 | 10 | 0.409 ±0.004**## | 67**## |
| 1 | 0.480 ±0.003**## | 79**## | |
| 10–1 | 0.520 ±0.005**## | 85**## | |
| 10–2 | 0.572 ±0.005**## | 94**## | |
| 10–3 | 0.609 ±0.005## | 100## | |
| 10–4 | 0.603 ±0.003## | 99## | |
| 10–5 | 0.598 ±0.004*## | 98*## | |
| 10–6 | 0.580 ±0.005**## | 95**## |
To investigate the possible protective effects of kaempferol, we evaluated PC-12 cell viability in the presence of Aβ25–35 at different concentrations. Approximately 78% of PC-12 cells survived after treatment with 20 μM Aβ25–35, and PC-12 cell viability increased to 97%, 94%, 89%, 81% and 80% when pretreated with 10–2, 10–3, 10–4, 10–5 and 10–6 μM kaempferol, respectively (Table IV). Therefore, these results showed that kaempferol could protect PC-12 cells from Aβ25–35-induced cell death and apoptosis in a dose-dependent manner. Subsequently, 10–3 μM kaempferol was used for further studies.
Table IV
Effects of kaempferol on activity of PC-12 cells damaged by Aβ25-35 (± SD, n = 6)
| Groups | Concentration [μmol/l] | Absorbance (OD) | Cell proliferation rate (%) |
|---|---|---|---|
| Blank | – | 0.614 ±0.009 | 100 |
| Control | – | 0.613 ±0.004** | 100** |
| Aβ25-35 | 20 | 0.481 ±0.011** | 78** |
| Kaempferol + Aβ25-35 | 10 | 0.465 ±0.012** | 76** |
| 1 | 0.477 ±0.008** | 78** | |
| 10–1 | 0.568 ±0.007**## | 93**## | |
| 10–2 | 0.598 ±0.008## | 97## | |
| 10–3 | 0.577 ±0.008*## | 94*## | |
| 10–4 | 0.547 ±0.006**## | 89**## | |
| 10–5 | 0.495 ±0.027** | 81** | |
| 10–6 | 0.491 ±0.027** | 80** |
We further tested the effects of fulvestrant (an ER antagonist) and U0126 (an ERK inhibitor) on PC-12 cell activity. The results showed that different concentrations of fulvestrant (0, 10–2, 10–1, 1, 10, 102 and 103 M) and U0126 (0, 10–3, 10–2, 10–1, 1, 10 and 102 M) had no significant effect on the proliferation of PC-12 cells (Tables V and VI). The final concentrations of fulvestrant and U0126 were 1 mol/l and 10 mol/l, respectively, and the proliferation rates were nearly 100.
Kaempferol inhibits PC-12 cell death through ER and ERK
PC-12 cell death was analysed by Hoechst/PI staining. The results showed that Aβ25–35 markedly increased the percentage of apoptosis compared with that of the blank group (p < 0.01). The addition of oestradiol and kaempferol markedly attenuated PC-12 cell apoptosis compared with that of the Aβ25–35 group (p < 0.01). However, with the addition of fulvestrant and U0126, the apoptotic rate of PC-12 cells was significantly increased (p < 0.01). This indicated that kaempferol might inhibit PC-12 Aβ25–35-mediated cell death through the ER and ERK signalling pathways (Figure 2).
Kaempferol regulates mRNA levels of genes related to apoptosis in PC-12 cells through ER and ERK
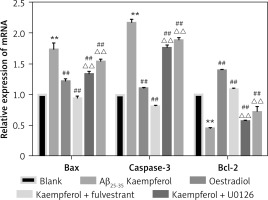
To further test our results, we measured the mRNA levels of genes related to cell apoptosis. The results showed that the expression of pro-apoptotic genes (Bax and caspase-3) was upregulated and that the anti-apoptotic molecule (Bcl-2) was downregulated in the Aβ25–35 group compared with those of the blank group (p < 0.01). Oestradiol and kaempferol significantly decreased the expression of the pro-apoptotic molecules (Bax and caspase-3) and increased the expression of the anti-apoptotic molecule (Bcl-2), whereas the antagonist fulvestrant and inhibitor U0126 partially reversed these effects of kaempferol (p < 0.01) (Figure 3).
Figure 3
Expression of genes related to apoptosis. Expression of apoptosis-related mRNA for Bcl-2, Bax and caspase-3 (active) was determined by RT-PCR. Data are presented as the mean ± SD (n = 3). Compared with blank group **p < 0.01; compared with Aβ25-35 group, ##p < 0.01; compared with kaempferol group, ΔΔ p < 0.01
Kaempferol regulates protein levels of genes related to apoptosis in PC-12 cells through ER and ERK
We further investigated the expression of related proteins. The results showed that the expression of pro-apoptotic genes (Bax and caspase-3) was upregulated and that the anti-apoptotic molecules (Bcl-2, ERβ and p-ERK1/2) were downregulated in the Aβ25–35 group compared with those of the blank group (p < 0.01). Oestradiol and kaempferol significantly decreased the expression of the pro-apoptotic molecules (Bax and caspase-3) and increased the expression of the anti-apoptotic molecules (Bcl-2, ERβ and p-ERK1/2) (Figure 4). The addition of the antagonist fulvestrant and inhibitor U0126 partly reversed the effects of kaempferol on Bax, caspase-3 and Bcl-2 (p < 0.01) (Figure 5 A). The PC-12 cells in the kaempferol group could still produce a certain amount of p-ERK1/2 when treated with Aβ25–35 only, while the addition of fulvestrant blocked p-ERK1/2 expression. The results suggest that kaempferol regulates apoptosis genes through the ER and ERK signalling pathways.
Figure 4
Expression of proteins related to apoptosis. Expression of the apoptosis-related proteins Bcl-2, Bax, caspase-3 (active) (A) and ERβ and p-ERK1/2 (B) was determined by Western blotting. Data are presented as the mean ± SD (n = 3). Compared with blank group, **p < 0.01; compared with Aβ25-35 group, ##p < 0.01
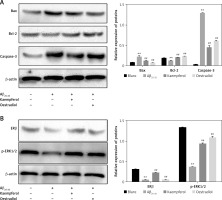
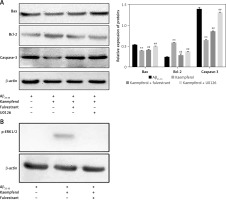
Figure 5
Effects of fulvestrant and U0126 on the kaempferol-mediated protection of PC-12 cells. Cells were exposed to 10 μM U0126 and 1 μM fulvestrant. Expression levels of Bcl-2, Bax, and caspase-3 (A) and ERβ and p-ERK1/2 (B) were evaluated by Western blotting. Data are presented as mean ± SD (n = 3). Compared with Aβ25-35 group, **p < 0.01; compared with kaempferol group, ##p < 0.01
Discussion
In the present study, we explored the effects of kaempferol on PC-12 cell apoptosis induced by Aβ25-35, using oestradiol as a positive control, and the underlying molecular mechanisms involved. Our results suggest that the inhibition of apoptosis by kaempferol is mediated, at least in part, through the ER/ERK/MAPK signalling pathway.
Alzheimer’s disease is a disease caused by the degeneration of the central nervous system [1, 29]. Many theories have been proposed regarding the pathogenesis of senile dementia, such as the amyloid theory, apoptosis theory, oxidative stress theory, calcium overload theory, and cholinergic theory [30–33]. Studies have shown that the development of AD is closely related to apoptosis [34]. Researchers have found that a large number of apoptotic neurons exist in the brains of AD patients and that programmed cell death plays an important role in central nervous development and the adult central nervous system [35]. Therefore, regulating apoptosis to prevent the occurrence of AD has received increasing attention.
Apoptosis is an important biological process that involves many aspects of biological growth and development. Apoptosis may be initiated by Bcl-2 family proteins, which consist of anti-apoptotic molecules (e.g., Bcl-2) and pro-apoptotic molecules (e.g., Bax), and involves mitochondrial release of cytochrome. This pathway may activate one or more of the initiator caspases. Kaempferol is a flavonoid found in many edible plants. Numerous preclinical studies have shown that kaempferol has a wide range of pharmacological activities, including antioxidant, anti-inflammatory, neuroprotective, oestrogenic/antiestrogenic, and analgesic activities. Nevertheless, little is known about the role of kaempferol in AD.
In our study, we chose different concentrations of kaempferol and oestradiol as a positive control. Aβ25–35 was used to establish AD models. The results showed that 10–3 μM oestradiol induced a clear protective effect on PC-12 cells against Aβ25–35 damage, and so this concentration was used for subsequent studies. Different concentrations of kaempferol markedly decreased the percentage of apoptotic cells and regulated the expression of genes and proteins related to apoptosis, upregulating the levels of anti-apoptotic molecules (Bcl-2, ERβ and p-ERK1/2) and downregulating the levels of pro-apoptotic molecules (Bax, caspase-3).
We further explored the signal transduction pathway involved in the protective effects of kaempferol against Aβ25–35-induced PC-12 cell apoptosis. Kaempferol can bind to both subtypes of the oestrogen receptor (ER), ER-α and ER-β. Among them, the affinity of kaempferol for the ER-β receptor is higher than for the ER-α receptor [36, 37]. Our data showed that the addition of the ER antagonist fulvestrant largely increased the apoptosis of PC-12 cells (which was inhibited by kaempferol). Moreover, fulvestrant decreased the expression levels of Bcl-2, ERβ and p-ERK1/2, which were increased by kaempferol and increased the levels of Bax and caspase-3, which were decreased by kaempferol. MAPK/ERK is one of the most important MAPK transduction pathways and plays a role in cell growth, development, differentiation and apoptosis [15].
Brain injury activates ERK1/2 and increases p-ERK1/2 to mediate the death of neurons. U0126, an ERK inhibitor, blocks the ERK/MAPK signalling pathway. Treatment with U0126 significantly increased the apoptosis of PC-12 cells and decreased the expression levels of anti-apoptotic molecules and increased the levels of pro-apoptotic molecules that were mediated by kaempferol. Therefore, we hypothesized that the ER/ERK/MAPK signalling pathway is involved in the protective role of kaempferol against Aβ25–35-induced PC-12 cell apoptosis.
In conclusion, the study suggests that kaempferol attenuates Aβ25–35-induced PC-12 cell apoptosis and that the ER/ERK/MAPK signalling pathway might be responsible for its protective effects. This study provides a theoretical basis for the treatment of AD, which still requires further clarification.