Introduction
In December 2019, a cluster of cases of pneumonia emerged in Wuhan City in Central China’s Hubei Province. Genetic sequencing of isolates obtained from patients with pneumonia identified a novel coronavirus as the etiology, now officially classified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Subsequently, the new disease has been officially named as coronavirus disease 2019 (COVID-19) by the WHO [2–4]. Since COVID-19 broke out in Wuhan City, SARS-CoV-2 injection has been pandemic in the whole world. As of July 16, 2020, a total of 85 697 confirmed cases have been identified and 4651 patients have died from SARS-CoV-2 infection in China. Moreover, more than 13.58 million confirmed cases and 581 229 death cases were found in other countries [5].
The clinical symptoms and features of COVID-19 patients include fever, dry cough, bilateral lung ground glass opacity, dyspnea and diarrhea. Some studies discovered that angiotensin-converting enzyme 2 (ACE2) was identified as a functional receptor for SARS-CoV-2 and was highly expressed in various organs [6, 7]. In severe and critically ill cases, SARS-CoV-2-caused pneumonia leads to not only severe acute respiratory syndrome but also multiple organ failure and even death [8]. Furthermore, several studies found that lymphocyte count from peripheral blood was decreased in most patients with COVID-19. Lymphocyte count in severe cases was obviously lower than that of mild cases [1, 8, 9]. Nevertheless, it remains unclear what factors lead to lymphopenia. The association between lymphopenia and the prognosis of COVID-19 patients needs to be determined. Therefore, we analyzed factors influencing lymphopenia as well as the association between lymphopenia and prognosis of COVID-19 patients in a hospital-based case-cohort study.
Material and methods
Subjects
In the present research, 220 cases confirmed with SARS-CoV-2 infection were enrolled from Union Hospital of Huazhong University of Science and Technology from January 1 to January 30, 2020. Union Hospital of Huazhong University of Science and Technology is one of the COVID-19-designated hospitals for the hospitalization of probable and confirmed COVID-19 cases in Wuhan City in central China’s Hubei Province. A medical team from the Second Affiliated Hospital of Anhui Medical University zealously went to Union Hospital and took part in medical treatment of COVID-19 cases in Wuhan City. We excluded cases with a negative SARS-CoV-2 RNA detection result, incomplete data or testing result. Children and pregnant women were also excluded from this research. Finally, 192 patients were included in this trial and 28 cases were eliminated. We reviewed and collected clinical electronic medical records including demographic data, clinical characteristics, comorbidities, nursing records and disease exposure history for all patients with laboratory confirmed SARS-CoV-2 infection. Biochemical indexes on admission and chest computed tomography (CT) were detected. We followed up the outcomes of COVID-19 patients. The clear prognostic indicators of COVID-19 patients included blood tests, liver function, renal function, myocardial function, respiratory function and chest CT. This study was approved by the institutional ethics board of the Second Affiliated Hospital of Anhui Medical University and Union Hospital of Huazhong University of Science and Technology. All participants or guardians gave oral informed consent.
Patient and public involvement
Patients were not directly involved in the development of the review update.
Date collection
Demographic data, laboratory results and CT image findings were acquired from medical records in the Union Hospital of Huazhong University of Science and Technology. The demographic data of all patients were collected: gender, age, smoking history, exposure history, comorbidities including cardiovascular disease, hypertension, chronic pulmonary disease, hepatic disease, diabetes and other chronic diseases (chronic pancreatitis, chronic gastritis, orthopedic diseases, etc.), symptoms and signs including cough, fever, fatigue and diarrhea. Biochemical indexes on admission included: blood tests, oxygen saturation, partial pressure of carbon dioxide (PaCO2) in artery, partial pressure of oxygen (PaO2), fraction of inspiration O2 (FiO2), PaO2/FiO2 ratio (oxygenation index), total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), total protein, albumin, globulin, albumin/globulin ratio, creatinine, urea nitrogen, uric acid, aspartate aminotransferase (AST), AST/ALT ratio, creatine kinase, lactate dehydrogenase, cardiac troponin T, D-dimer, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP).
All patients underwent chest CT scan. All CT images were analyzed by two independent radiologists with rich clinical experience. All images were viewed on both lung (width, 1500 HU; level, –700 HU) and mediastinal (width, 350 HU; level, 40 HU) settings. The presence or absence of all image features was recorded: ground-glass opacities, consolidation, traction bronchiectasis, bronchial wall thickening, reticulation, subpleural bands, vascular enlargement and lesion distribution. The detailed CT image characteristics of 200 COVID-19 patients were described in another work [10]. On the image scans, the pulmonary tissues were divided into two single lungs (left and right lung) and three zones (upper, middle, and lower zones of lung in the bilateral lungs). These areas of the lung were defined as the “upper zones” above the level of the carina; the “middle zones” between the level of the carina and the level of the inferior pulmonary veins; and the “lower zones” below the level of the inferior pulmonary veins. The CT score was determined by visually estimating the extent of disease in each zone. The severity scores were evaluated according to the percentage of lung parenchyma in each zone that showed evidence of each recorded abnormality: (1) no injury; (2) 1 point, involvement of less than 25% of the image; (3) 2 points, 25% to 50%; (4) 3 points, 50% to 75%; (5) 4 points, more than 75%. Total severity scores (between 0 and 24) for each lung were generated by adding all the partial areas. The total scores represented the damage area for each lung tissue [11].
Statistical analysis
All data were statistically analyzed with SPSS 21.0 software. Categorical variables were expressed with frequencies and percentages. Data were expressed as mean or median for the continuous variables. Means for continuous variables were compared with independent-samples t tests when the data were normally distributed; if not, the Mann-Whitney test was used. The comparison of discrete variables between different groups was evaluated using the χ2 test and Fisher’s exact test. Moreover, the main risks related with lymphopenia were examined using a multivariable logistic regression model. Statistical significance was determined at p-values of less than 0.05.
Results
Demographic data and clinical characteristics among COVID-19 patients
All the 192 patients (50.0% males) aged between 22 and 87 years were included in the analysis. The normal range of lymphocyte count in the peripheral blood is from 0.8 to 4 (109/l). Lymphopenia (lymphocyte count < 0.8 × 109/l) was present in most COVID-19 patients. The demographic and clinical characteristics of all COVID-19 patients are shown in Table I. Patients with high and low counts of lymphocytes (split at the low thresholds, 0.8 × 109/l) were not different in gender or comorbidities including diabetes, arterial hypertension, hepatic disease, cardiac disease, pulmonary disease and other chronic disease (chronic pancreatitis, chronic gastritis, orthopedic diseases). Clinical symptoms, such as fever, diarrhea, fatigue and cough, as well as injured pulmonary nodes (bilateral lungs, single left lung, single right lung), were not different in COVID-19 patients with normal lymphocyte and lymphopenia (Table I). Nevertheless, the number of patients over 70 years old with lymphopenia was higher than the number of patients over 70 years old with normal lymphocytes (24 (28.6%) vs. 17 (15.7%)).
Table I
Demographics and baseline characteristics of patients with COVID-19
Older COVID-19 patients are more susceptible to lymphopenia
The blood tests of all COVID-19 patients on admission to hospital were measured and the number of abnormal cases was calculated. We found that in 84 (43.8%) cases lymphocyte count was decreased and in 97 (50.8%) cases lymphocyte percentage was decreased. In 64 (33.5%) cases white blood cell (WBC) count was under the normal range. In 105 (55.2%) cases eosinophil count and in 120 (62.5%) eosinophil percentage were below the normal range. In 114 (59.4%) neutrophil percentage was higher than the upper limit (data not shown). The effects of different gender on lymphocyte count and lymphocyte percentage were analyzed. As shown in Table II, lymphocyte counts were similar in male and female patients with COVID-19. Lymphocyte percentage in males was obviously lower than that in females (16.4 vs. 22.3%, p = 0.012). The effects of age on lymphocyte count and lymphocyte percentage were then evaluated. The lymphocyte counts in patients over 70 years old were evidently lower compared with those under 60 years old and from 60 to 69 years old (0.66 vs. 0.92 with 0.92, p = 0.012). Similarly, the lymphocyte percentage in patients over 70 years old was lower than in those under 60 years old and between 60 to 69 years old (12.1% vs. 22.1% with 15.8%, p = 0.001). Also, there were no differences of lymphocyte count or lymphocyte percentage between smokers and non-smokers. Among all patients, 155 (80.7%) had at least one comorbidity, such as 129 (67.2%) diabetes, 96 (50.0%) hypertension, 9 (4.69%) hepatic disease, 16 (8.33%) cardiac disease, 8 (4.17%) pulmonary disease and 7 (3.65%) other chronic diseases. The impacts of comorbidities on lymphocyte count and lymphocyte percentage were then analyzed. There was no significant difference of lymphocyte count or lymphocyte percentage between COVID-19 patients with and without diabetes, hypertension, hepatic disease and other chronic diseases (Table II). Interestingly, we found that lymphocyte percentage in patients with cardiac disease and pulmonary disease was prominently lower than that in patients without cardiac disease and pulmonary disease (12.0% vs. 20.6%, p = 0.028; 10.6% vs. 20.1%, p = 0.045). Multivariable logistic regression was used to analyze risk factors of lymphopenia in 192 patients with COVID-19. As shown in Table III, the OR of lymphopenia in patients from 60 to 69 years old was 0.290 (95% CI: 0.096–0.874; p = 0.028), and the OR of lymphopenia in patients under 70 years old was 0.350 (95% CI: 0.124–0.989; p = 0.048). No significant correlation was observed between gender, smoker status and comorbidities with lymphopenia in COVID-19 patients (Table III).
Table II
Count and percentage of lymphocytes on admission among COVID-19 patients
Table III
Multivariable logistic regression between demographic data and lymphopenia among COVID-19 patients on admission
Lung damage is more serious among COVID-19 patients with lymphopenia
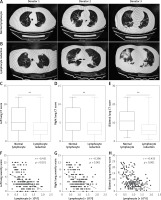
Chest CT was examined among COVID-19 patients. As shown in Figure 1 A, mild lung markings and patch clouding lung ground glass shadow in the subpleural area of bilateral lungs were found in COVID-19 patients with normal lymphocytes. By contrast, increased pulmonary bronchovascular shadows, indistinct lung markings and blur, pulmonary consolidation and nodules, and extensive high-density area with obvious lung ground glass shadow in the bilateral lungs were the main lung manifestations in COVID-19 patients with lymphopenia (Figure 1 B). Also, the severity of lung damage was scored by two experienced radiologists blind to the clinical data. As can be seen in Figures 1 C–E, the results showed that left lung CT score, right lung CT score and bilateral lungs CT score were all evidently increased in patients with lymphopenia (5.0 vs. 3.0, 6.0 vs. 3.0, 11.0 vs. 6.0). Further correlation analysis indicated that lymphocyte count was significantly and negatively associated with CT scores of left lung, right lung and bilateral lung (r = –0.341, p < 0.001; r = –0.396, p < 0.001; r = –0.435, p < 0.001), respectively.
Figure 1
CT images and the association between lymphopenia and CT scores. A – CT images from three different patients with normal lymphocytes are shown. B – CT images from three different patients with lymphopenia are shown. C–E – CT scores of left lung, right lung and bilateral lung were evaluated. F–H – Associations between lymphocyte count and CT scores in left lung, right lung and bilateral lung were analyzed among COVID-19 patients (n = 192). **P < 0.01
Multiple organ injuries are more serious among COVID-19 patients with lymphopenia
The effect of lymphopenia on admission on extrapulmonary organ multiple organ injures was analyzed in COVID-19 patients. Hepatic function related biochemical indexes on admission were detected among all COVID-19 patients. As shown in Table IV, TBIL and DBIL in patients with lymphopenia were obviously higher than those in patients without lymphopenia. The level of ALT was not different between the two groups. Compared with patients with lymphopenia, total protein and albumin were lower than those in patients without lymphopenia. There was no difference in globulin or albumin/globulin ratio between two groups. Renal functions were also measured on admission among COVID-19 patients. As shown in Table IV, creatinine and urea nitrogen were increased in patients with lymphopenia. No significant difference in uric acid was observed between the two groups. Moreover, myocardial function results suggested that creatine kinase, lactate dehydrogenase (LDH), AST and AST/ALT ratio were significantly increased in patients with lymphopenia compared with values in patients without lymphopenia. The numbers of myoglobin-positive and cardiac troponin T-positive patients with lymphopenia were higher than in patients with normal lymphocytes. The number of creatine kinase isoenzymes-positive patients were equal between the two groups. Additionally, respiratory function related biochemical indexes of COVID-19 patients were evaluated on admission. PaCO2 (33.5 mm Hg vs. 39.0 mm Hg), SpO2 (74.0% vs. 94.0%), and oxygenation index (237.7 vs. 338.1) were remarkably decreased in patients with lymphopenia. In addition, we found that D-dimer and erythrocyte sedimentation rate were prominently increased in patients with lymphopenia.
Table IV
Biochemical indexes on admission to hospital of COVID-19 patients
Lymphopenia on admission elevates disease severity of COVID-19 patients
Among all patients, severe and critically ill cases, defined as oxygenation index lower than 300, accounted for 111 (57.8%). Mild cases, oxygenation index higher than 300, accounted for 81 (42.2%). The relationship between lymphocyte count and the severity of COVID-19 was analyzed. As shown in Table V, severe or critically ill patients numbered 59 (72.0%) among patients with lymphopenia, and 43 (41.0%) among patients with normal lymphocytes. The RR was 1.757 (95% CI: 1.346–2.292; p < 0.001) in severe and critically ill COVID-19 patients with lymphopenia. When the patients were more than 60 years old, the severe or critically ill patients numbered 38 (82.6%) among patients with lymphopenia, and 16 (33.3%) among patients with normal lymphocytes. The RR was 2.478 (95% CI: 1.626–3.777; p < 0.001) in severe and critically ill COVID-19 patients with lymphopenia. When the patients were below 59 years old, there was no difference of disease severity between lymphopenia and normal lymphocytes. Additionally, the effects of lymphocyte count and comorbidities on death risk were analyzed. As shown in Table V, when the patients had comorbidities, the RR was 1.647 (95% CI: 1.226–2.212; p = 0.001) in severe and critically ill COVID-19 patients with lymphopenia. When the patients had not comorbidities, the RR was 3.333 (95% CI: 1.594–6.970; p = 0.001) in severe and critically ill COVID-19 patients with lymphopenia.
Table V
Relationships of lymphopenia with severity and death risk in COVID-19 patients
Lymphopenia on admission elevates the death risk of COVID-19 patients
The effect of lymphopenia on admission on the fatality rate was evaluated. As shown in Table V, 32.1% (27/84) died among COVID-19 patients with lymphopenia, whereas only 5.56% (6/108) died among patients without lymphopenia. Further analysis showed that lymphopenia elevated death risk of COVID-19 patients. The RR was 5.789 (95% CI: 2.504–13.367; p < 0.001) in COVID-19 patients with lymphopenia. When the patients were below 59 years old, 18.4% (7/38) died among COVID-19 patients with lymphopenia, whereas only 3.33% (2/60) died among patients without lymphopenia. Further analysis showed that lymphopenia elevated the death risk of COVID-19 patients. The RR was 5.526 (95% CI: 1.211–25.218; p = 0.012) in COVID-19 patients with lymphopenia. When the patients were more than 60 years old, the dead patients numbered 24 (52.2%) among patients with lymphopenia, and 4 (8.33%) among patients with normal lymphocytes. The RR was 6.261 (95% CI: 2.354–16.652; p < 0.001) for death risk of COVID-19 patients with lymphopenia. Moreover, we found that 24 (33.3%) patients died among patients with lymphopenia and comorbidities, while 6 (7.23) died among patients with lymphopenia and comorbidities; the RR was 4.611 (95% CI: 1.997–10.650; p < 0.001) for death risk of COVID-19 patients with lymphopenia. In addition, when patients had no comorbidities, there was no difference in death risk between patients with lymphopenia and those with normal lymphocytes.
Discussion
The present research analyzed the association between lymphopenia at the early stage and prognosis of COVID-19 patients in a hospital-based case-cohort study. The main findings of the present research include the following: older COVID-19 patients are more susceptible to lymphopenia; multiple organ injuries were more serious in COVID-19 patients with lymphopenia; lymphopenia at the early stage aggravates the severity and elevates the death risk of COVID-19 patients.
Several studies have found that lymphocyte count was decreased in COVID-19 patients [1, 9, 12, 13]. In addition, several reports have shown that lymphocyte count was lower in severe COVID-19 cases than in mild ones [14]. It remains obscure what factors lead to lymphopenia on admission among COVID-19 patients. In the present research, we analyzed the relationship between age and lymphopenia among COVID-19 patients. Our results showed that in almost half of patients lymphocyte count and percentage were reduced to below the normal range. Moreover, the number and percentage of lymphocytes were lower in COVID-19 patients over 70 years old than those of younger patients. The effects of gender and comorbidities on lymphopenia were analyzed among COVID-19 patients. Our results showed that lymphocyte percentage was obviously lower in males than in females. Of interest, lymphocyte percentage was reduced only in COVID-19 patients with either cardiac diseases or pulmonary chronic diseases. To control possible confounding factors, multivariable logistic regression was used to further analyze the influence of gender, age and comorbidities on lymphopenia in COVID-19 patients. We found that older age was the major risk factor of lymphopenia on admission among COVID-19 patients. Our results suggest that lymphocytes may be a direct target of SARS-CoV-2. Elderly patients are more likely to suffer from lymphopenia at the early stage of SARS-CoV-2 infection.
Previous studies found that multiple organ injures were prevalent in almost all COVID-19 patients, especially in the critically ill [15–17]. In the present research, we evaluated the severity of lung injury in COVID-19 patients using CT scores. We observed obvious lung damage in almost all COVID-19 patients. Moreover, the CT scores were higher in COVID-19 patients with lymphopenia than those in COVID-19 patients with normal lymphocytes. Correlation analysis revealed that lymphocyte counts were negatively associated with CT scores. The association between lymphopenia and respiratory function indices was then analyzed among COVID-19 patients. As expected, PaCO2, SpO2 and oxygenation index, respiratory function markers, were decreased in COVID-19 patients with lymphopenia. Next, the association between lymphopenia and extrapulmonary organ injuries was analyzed among COVID-19 patients. We found that TBIL and DBIL, two markers of hepatic injury, creatinine and urea nitrogen, two indices of renal function, and creatine kinase, AST and LDH, three myocardial enzymes, were obviously elevated in COVID-19 patients with lymphopenia. These results suggest that lymphopenia at the early stage may be a potential indicator of multiple organ injures among COVID-19 patients.
Several reports have demonstrated that lymphopenia was more obvious in critically ill patients [18–21]. However, the relationship between lymphopenia at the early stage and the severity and death risk of COVID-19 patients has not yet been determined. Our results showed that the severity of COVID-19 was positively associated with lymphopenia on admission. Moreover, fatality rate was higher in patients with lymphopenia than in patients with normal lymphocytes. Further stratified analysis found that regardless of age, the death risk was elevated in patients with lymphopenia. Furthermore, whether with or without comorbidities, the severity was increased among COVID-19 patients. These results indicate that lymphopenia elevates the severity and death risk of COVID-19 patients, which explains why the fatality rate was higher in older COVID-19 patients than in younger ones. The present study provides evidence that lymphopenia on admission may be a potential negative prognostic indicator for COVID-19 patients.
In brief, this study analyzed factors influencing lymphopenia as well as the association between lymphopenia and prognosis of COVID-19 patients in a hospital-based case-cohort study. Nevertheless, there are several limitations in this research. Firstly, because of the small sample size, analysis of larger sample sizes is required in future work. Secondly, the cases were enrolled from one hospital in China, so the results may not generalizable to COVID-19 patients from all over the world; multicenter research is needed. Thirdly, the causal relationship between lymphopenia and multiple organ damage was not clear; additional longitudinal studies and animal experiments could answer the question. Fourthly, stratified analysis revealed that age and comorbidities have a certain effect on lymphopenia-aggravated severity and death risk among COVID-19 patients. Therefore, age and comorbidities may be confounding factors and cannot be fully excluded in the present cohort study.
In conclusion, the present study analyzed the association between lymphopenia at the early stage and prognosis of COVID-19 patients. We found that older patients were more susceptible to lymphopenia. Moreover, lymphopenia on admission was positively associated with multiple organ injuries. In addition, lymphopenia on admission elevated the severity and death risk of COVID-19 patients. Our results suggest that lymphocytes may be a direct target of SARS-COV-2. We provide evidence that lymphopenia at an early stage may be a potential negative prognostic indicator for COVID-19 patients. Therefore, surveillance of lymphocyte count is helpful for the early screening, diagnosis and treatment of critically ill COVID-19 patients.