Severe sepsis and septic shock are the most common causes of death in the intensive care unit (ICU), with a mortality rate of about 25–30%. Capillary leak (CL) with progressive subcutaneous, pulmonary, and body-cavity oedema typically develops in patients with sepsis, septic shock, and pancreatitis.In these patients, hypoalbuminaemia, caused by poor nutritional status, haemodilution, and disturbed hepatic synthesis, further provoke fluid shifts [1–3]. CL and positive fluid balances are known to be independent predictors of morbidity and mortality in ICU patients [4–8].
Recently, a non-invasive technique, called bioelectrical impedance analysis (BIA), was explored to measure the body fluid compartments and body composition in critically ill patients [9, 10]. During BIA, a low-level battery current passes through the body, and the impedance, phase angle, resistance, and reactance of the body are measured. Based on these measurements, pre-programmed mathe-matical algorithms calculate body fluid compartment volumes (total body water, intra- and extracellular water volumes, volume excess), muscle mass, and fat-free mass. BIA can be carried out as a full-body measurement or as a segmental assessment [11, 12].
Originally, BIA was developed as a tool to evaluate hydration status in patients with acute heart failure and chronic haemodialysis and to follow up nutritional status in cancer patients or during long-term parenteral nutrition. Recently, its applicability as a tool to assess fluid status in critically ill patients has been explored [9, 10, 13]. This application is attractive because BIA is a non-expensive, non-invasive, and bedside-applicable tool to guide medical decisions concerning fluid management in intensive care patients [14, 15].
However, evidence on the reproducibility of BIA measurements is scarce. It is unclear whether differences in results should be linked to differences in the patient’s clinical status or to reproducibility issues. Limb dominance might affect results when measured on the non-dominant <i>versus</i> the dominant body side [16–19]. Moreover, it is not clear if variability between different measurements at different points in time is acceptable with newer multiple-frequency and segmental BIA methods [12]. However, reproducibility is essential for correct interpretation of the results. Therefore, the objective of this study was to evaluate the reproducibility of BIA-derived parameters by comparing non-dominant <i>versus</i> dominant body-side measurements, and by evaluation at different time-points in healthy volunteers. We assumed that healthy volunteers have a similar fluid status on both body sides and over several days.
MATERIAL AND METHODS
This prospective validation experiment was carried out in 22 healthy volunteers in September 2015. The study protocol was approved by the Ethics Committee Research UZ/KU Leuven. Procedures were carried out in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Volunteers were not eligible for inclusion if they had a pacemaker or an automatic defibrillator, were pregnant women, and had a limb amputation or carried a prosthesis, or any type of orthopaedic implant. Volunteers were allowed to continue their regular food and beverage intake; they were asked not to perform any strenuous exercise on the day of measurement. Before measurement, volunteers were first asked to rest on a bed in a supine position for 10 minutes, arms by their side and separated from the trunk, and legs separated from each other, as described by Kyle et al. [11] and the manufacturer. BIA measurements were then carried out in the same position using the Bioscan 920-II (multiple frequencies, 5–50–100–200 kHz; Maltron, Rayleigh, Essex, UK) and following the manufacturer’s instructions. Analyses started on the left body side and were immediately followed by a measurement on the right body side, both as full-body and segmental (whole arm, upper arm, lower arm, torso, whole leg, upper leg, and lower leg) measurement. Tetrapolar measurements were performed using 8 electrodes, arranged in a standardized configuration [11]. Two emitting electrodes were placed on the dorsal surface of the hand and foot. For whole-body measurements sensing electrodes on the dorsum of the wrist and the anterior surface of the ankle were used. For segmental measurements, additional sensing electrodes on the proximal portion of the forearm and the lower leg, the shoulder and the upper thigh were used. Limb dominance reported by the volunteers was documented.
To assess reproducibility of BIA in function of time, volunteers were measured on 2 separate days within 1 week. The measurements were carried out on one occasion on each of the 2 days, regardless of timing and amount of food and beverage intake. The parameters of interest were both the raw data (impedance and phase angle) at the individual frequencies (5–50–100–200 kHz) and the (final) body fluid compartment volume estimations: total body water (TBW [L]), extracellular water volume (ECW [L]), intracellular water volume (ICW [L]), and volume excess. Concerning the raw data, resistance and reactance were not separately analysed because impedance is a resultant of both.
The endpoints were the reliability and potential significant differences in the abovementioned measurements on the non-dominant <i>versus</i> the dominant side, and as a function of time. We assumed healthy volunteers to be in homeostasis. Hence, the reliability between BIA measurements should be high, and the differences should not be significant.
The reliability of the measurements was assessed by performing a single measurement, absolute agreement, and 2-way mixed-effects model to calculate the intraclass-correlation coefficient (ICC, [95% confidence interval (CI)]). Based on the manufacturer’s specifications and current guidelines, a minimal ICC of 90% is required for excellent reliability [20–22]. Significant differences were checked for by performing a paired Student’s t-test or a paired Wilcoxon rank sum test, depending on a normal distribution of the measurements. A 2-sided significance level was set at 0.05. All statistical tests were performed using R software (R version 3.5; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
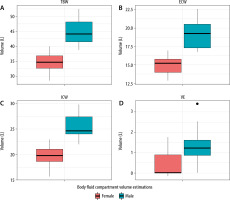
A total of 22 volunteers were included on 42 matched occasions (2 volunteers were absent for the second analysis). Volunteers’ demographic characteristics are depicted in Table 1. Body fluid compartment volume estimations from full-body analysis for men and women are illustrated in Figure 1. Results for full-body BIA and 2 illustrations of segmental analyses (i.e. upper arm and torso) are shown in Tables 2 and 3, respectively. Median values and interquartile range are presented for each estimated body fluid compartment volume and each of the raw measurements at the individual frequencies.
TABLE 1
Demographic characteristics (N = 22)
| Characteristics | |
|---|---|
| Length, cm | 172 ± 9 |
| Body mass, kg | 64 (59; 68) |
| Body mass index, kg m–2 | 22.09 ± 3.95 |
| Age, years | 26 (24; 35) |
| Gender – men (%) | 32 |
| Time between measurements, days | 2.07 (1.00; 2.99) |
TABLE 2
Reliability for full-body bioelectrical impedance analysis
a Intraclass correlation coefficient non-dominant versus dominant side. bP-value calculated by paired t-test or Wilcoxon rank sum test for non-dominant versus dominant side. cIntraclass correlation coefficient in function of time. dP-value calculated by paired t-test or Wilcoxon rank sum test in function of time
TABLE 3
Reliability for segmental bioelectrical impedance analysis: upper arm and torso
a Intraclass correlation coefficient non-dominant versus dominant side; bp-value calculated by paired t-test or Wilcoxon rank sum test for non-dominant versus dominant side, cIntraclass correlation coefficient in function of time; dp-value calculated by paired t-test or Wilcoxon rank sum test in function of time; eNot applicable because volume excess is 0 for all measurements
FIGURE 1
Body fluid compartment volume estimations (TBW – total body water, ECW – extracellular water volume, ICW – intracellular water volume, VE – volume excess) from full body bioelectrical impedance analysis for males and females
Full-body analysis
As presented in Table 2, for all body fluid compartment volume estimations from full-body analysis, ICCs for the non-dominant <i>versus</i> dominant side measurements were greatly above 90%, except for volume excess showing moderate agreement. Moreover, differences were not significant, except for ICW. For body fluid compartment volume estimations over time, ICCs were also greatly above 90%, except for volume excess, and showed no significant differences. Raw impedance and phase angle measurements showed variable and low ICCs and significant differences, both for results comparing non-dominant <i>versus</i> dominant side measurements and results over time.
Segmental analysis
For upper arm analysis, although significant differences were only seen for the body fluid compartment volume estimations over time, ICCs dropped below 90% for both the non-dominant <i>versus</i> dominant side measurements and those over time (Table 3). For torso analysis, non-dominant <i>versus</i> dominant side measurements and those over time showed variable and low ICCs, and significant differences (Table 3). Similarly, all other segmental results (whole arm, lower arm, whole leg, upper leg, lower leg) demonstrated variable and low ICCs (results not shown).
DISCUSSION
In this validation study, we showed that body fluid compartment volumes estimated by full-body BIA could be reproduced with excellent reliability between non-dominant <i>versus</i> dominant side measurements and over time, in healthy volunteers. However, in both cases, raw impedance and phase angle measurements at the individual frequencies (i.e. 5–50–100–200 kHz) showed insufficient reliability. As shown by Ward et al. [19], limb dominance has the potential to cause significant differences in raw full-body BIA measurements between both body sides. Our results show that, despite significant alterations in raw measurements, underlying mathematical calculations correct for this when performing full-body BIA. The Maltron Bioscan 920-II calculates the body fluid compartment volume estimations via a “black-box” algorithm, which incorporates the raw data, together with data inserted by the user before analysis, in mathematical equations unknown to the user. Because algorithms are not made publicly available to the user for many commercially available devices, the user should be careful when using volume estimations calculated by specific devices. Hence, this study presents evidence for reliable and reproducible conclusions for full-body fluid compartment volume estimations when using the Maltron Bioscan 920-II.
On the one hand, because we assumed that healthy volunteers have a similar fluid status on both body sides, these results proved the reproducibility of the BIA device for estimating full-body compartment volumes on both body sides in this population. Based on our validation study, when performing full-body BIA in ICU patients, real differences in fluid status will thus be detected. Importantly, users need to be aware that in ICU patients, both body sides might show different BIA results because there might be tissue oedema [23, 24]. Additionally, conductance might be altered at one of the body sides due to the presence of (arterial or venous) catheters, etc. Lingwood et al. [25] detected significant interference from cardiorespiratory monitors in adult and neonate ICU patients. However, this interference can be minimized by using multiple frequency measurements. Dewitte et al. [26] were able to document the absence of an effect of ICU monitoring systems and mechanical ventilation on the determination of fluid status using bio-impedance spectroscopy in adults. Nevertheless, the influence of arterial or venous catheters, other electrical monitoring equipment, and the ICU environment has not yet been excluded. Moreover, limb dominance can cause additional discrepancies between both body sides. Based on this, in our opinion, it is recommended that body fluid compartment volumes be assessed in critically ill patients by applying BIA to both body sides, instead of relying only on a one-sided result. Another option to overcome these issues may be to use the “STAR” algorithm, which collects triple raw data at the left and right side as well as data obtained from left to right and finally calculating an overall average. This provides a robust measurement that is reproducible also when unilateral differences occur.
On the other hand, full-body fluid compartment volumes did not differ significantly in healthy volunteers, who were assumed to be in homeostasis, when measured over several days. Baldwin et al. [27] also found excellent test-retest (after 2 days) reli-ability for full-body fluid compartment volumes, using bioelectrical impedance spectroscopy in healthy volunteers and critically ill patients. How-ever, they only recorded raw measurements at 50 kHz and did not assess ICC. Several other publications focused on comparing measures of body composition calculated by BIA (e.g. body fat, muscle mass) compared to reference methods, rather than specifically looking at the reproducibility of the measurements [27–29].
Based on this study, future discrepancies in full-body BIA measurements, when adopted in clinical practice in the ICU, will be related to differences or changes in a patient’s clinical status and are not induced by the device itself.
The reproducibility of the segmental BIA results is unclear because variable and lower reliability was observed in healthy volunteers, both for non-domi-nant <i>versus</i> dominant side measurements and for measurements over time. The cohort of healthy volunteers was young (median age 26 years), they were not taking any fluid balance impacting medication, and they were considered to be in homeostasis, so changes are challenging to interpret. We presume that these results may reflect anatomical differences (e.g. differences in water distribution in the legs depending on walking or resting all day, differences in the muscularity of the non-dominant <i>versus</i> dominant upper arm) and possibly normal daily variation in bioelectrical impedance [30]. On the other hand, the discrepancies between non-dominant and dominant side, and over time, might point out that the BIA device is not accurate for the measurement of smaller body segments. Moreover, when interested in the whole-body composition, segmental analysis is not better than full-body analysis [31]. Therefore, the clinical meaning of using segmental BIA in the ICU cohort is not well understood, and we would recommend performing full-body BIA measurements on both body sides, because this provides reliable information on the fluid status.
A few limitations have to be taken into account. We included only 22 healthy volunteers. Although this is a relatively small sample size, results for full-body BIA are evident. Also, several conditions could influence the reproducibility of the BIA measurement. Previous exercise, dietary intake, skin temperature, body position, and electrode position have been shown to interfere with bioelectrical impedance results [12, 32]. However, we tried to standardize the measurement conditions as much as possible (e.g. no strenuous exercise prior to measurement, 10 minutes of resting in a supine position prior to measurement, standardized electrode configuration). Moreover, even though we do not expect significant changes in body weight over a couple days in healthy volunteers, we were not able to confirm this because their body weight was not measured on a second occasion. Finally, we did not perform measurements on different occasions during the same day. However, we performed measurements on 2 different days within 1 week, regardless of food or beverage intake.
CONCLUSIONS
In this validation study, no significant changes have been observed in body fluid compartment volumes assessed by full-body BIA in a healthy volunteer cohort, neither between non-dominant and dominant side measurements nor between 2 measurements over several days. Raw measurements and segmental BIA failed to show sufficient reproducibility. Based on the results of this study and current literature, when monitoring fluid status in the ICU patient, we recommend using body fluid compartment volumes estimated by full-body BIA on both body sides. This method allows for reliable monitoring of changes or differences in fluid status.




