Introduction
IgG4-related disease (IgG4-RD) is a potentially systemic disease, characterized by high serum IgG4 levels and multi-organ infiltration [1]. All organs and systems may be involved, mimicking many other conditions and more common diseases, such as systemic lupus erythematous, rheumatoid arthritis (RA), or anti-phospholipid syndrome [1].
Involvement of the salivary and lacrimal glands, previously called Mikulicz’s disease, is now reclassified as IgG4-related dacryoadenitis and sialadenitis (IgG4-DA/SA); similarly, Küttner tumour, unilateral swelling of the submandibular gland, is now called IgG4-related submandibular gland disease [2].
Once considered a subtype of Sjögren’s syndrome (SS), these 2 conditions should be also distinguished according to their histological findings. More in detail, IgG4-RD is characterized by tissue fibrosis with a storiform pattern, obliterative vasculitis, tissue eosinophilia, and lymphoplasmacytic infiltration with a prevalence of IgG4-positive plasma-cells and an IgG4/IgG ratio > 40% [3].
It should be stressed that IgG4+ plasma cells can be found in other conditions such as many autoimmune diseases, malignancies, and infections, while the abovementioned histological findings are more specific for IgG4-RD [4].
Nevertheless, differential diagnosis between the primary SS (pSS) and IgG4-RD can be challenging due to their similar clinical presentation; moreover, the coexistence of both IgG4-RD and pSS was also hypothesized and first reported in a Japanese patient fulfilling the diagnostic criteria for both diseases [5–7].
The aim of our study was to assess the prevalence of IgG4-SA in a cohort of Caucasian patients presenting with xerostomia, and to evaluate the presence of an overlap between IgG4-SA and SS.
Material and methods
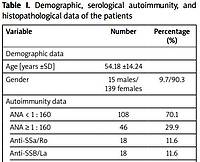
A total of n = 154 Italian Caucasian patients (15 ma-les, 139 females) was evaluated. Mean age was 54.18 ±14.24 years. We included all patients who consecutively underwent minor salivary gland biopsy (MSGB) in our department due to suspicion of SS caused by xerostomia, from March to December 2019. Exclusion criteria were active hepatitis C virus, human immunodeficiency virus, tuberculosis, sarcoidosis, end-stage renal disease, and previous head and neck radiotherapy, as well as any sign or symptom of extra-glandular involvement.
We used the punch biopsy technique proposed by Guevara-Gutierrez et al. [8], using a 4-mm punch and 4–0 absorbable sutures, following local anaesthetic infiltration with lidocaine. Salivary gland samples were subsequently fixed in formalin and evaluated by the pathology department of our university using ChisholmMason (CM) and focus score (FS) for SS and immunohistochemical study with IgG4 staining for IgG4-SA.
Histological report was considered putative for SS when CM ≥ 3 and/or FS ≥ 1, putative for IgG4-SA when > 10 IgG4 per high-power field (HPF) were detected, as well as an IgG4/IgG ratio ≥ 40. Anti-nuclear antibodies (ANA), anti-extractable nuclear antigens (ENA) with fluorescent enzyme immunoassay (FEIA), anti-double stranded DNA (dsDNA, FEIA method) antibodies, rheumatoid factor (RF), and anti-citrullinated protein antibodies (ACPA, FEIA method) were concomitantly assessed, as well as serum cryoglobulins and protein electrophoresis.
The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) classification criteria were used for the definitive diagnosis of pSS [9], and comprehensive criteria by Umehara et al. [10] for IgG4-SA, because, to date, no organ-specific criteria have been proposed [10].
Results
Anti-nuclear antibodies titre < 1 : 160 was found in 108 patients, 1 : 160 in 12, 1 : 320 in 17, 1 : 640 in 13, > 1 : 640 in 4, and ENA screening was positive (ratio > 1) in 11.7% (n = 18 patients) corresponding to the positivity of anti-Ro/SSA and anti-La/SSB antibodies.
The results of dsDNA (> 15 IU/ml), RF and ACPA (> 7 IU/ml) were positive in 2, 3, and 3 patients, respectively. Only one patient had cryoglobulins (mixed polyclonal, cryocrit 0.5%), while hypergammaglobulinaemia (> 20%) was found in 6 subjects. 44.8% (n = 69) had FS 0, and FS ≥ 1 was found in 55.2% (n = 85), while CM < 3 and CM ≥ 3 were seen in 47.4% (n = 73) and 52.6% (n = 81), respectively.
An IgG4/HPF value of 20 was found in 3 SS patients (1.9%), but an IgG4/IgG ratio ≥ 40 was found in none of them, nor tissue fibrosis with storiform pattern, obliterative vasculitis, or tissue eosinophilia. A definite diagnosis of primary Sjögren’s syndrome (pSS), using histological findings and/or immunological results, was made in 59.74% (n = 92) out of 154 patients who underwent MSGB.
In 3 cases, an overlap between SS and RA was present. The pSS negative group was diagnosed as “nonspecific sicca syndrome” and was not followed up in time. In the 2-year follow-up regarding the pSS-positive group, one case of IgG4 pulmonary-related disease was diagnosed, while one patient developed lymphoma. There were no patients who received a definite diagnosis of IgG4-SA. The obtained data are presented in Table I.
Table I
Demographic, serological autoimmunity, and histopathological data of the patients
Discussion
To our knowledge, our study represents the first systematic attempt to evaluate IgG4 infiltration in an Italian-based Caucasian cohort of patients who underwent MSGB for xerostomia. In our cohort, 92 patients (59.74%) received a diagnosis of pSS, while none fulfilled the diagnostic criteria for IgG4-SA.
Curiously, one patient, previously diagnosed with pSS, was subsequently evaluated for an interstitial lung disease, and a lung biopsy led to a diagnosis of IgG4-related respiratory disease. Such incidence is notably and surprisingly lower than reported in the Japanese study by Yamamoto et al. [11] in whch 7 of 160 evaluated patients (4.4%) were affected by IgG4-SA, with 6 of them presenting anti-Ro/SSA positivity.
On the other hand, in a Chinese cohort in which an overlap syndrome was suspected, 36 out of 336 pSS patients (10.7%) showed IgG4/IgG ratio ≥ 40% and > 10 IgG4 plasma cells/HPF. Such histological findings were positively associated with interstitial lung disease and tubulointerstitial nephritis, but negatively correlated with anti-Ro/SSA antibodies, anti-La/SSB antibodies, ANA, and RF, showing less lymphocytic infiltration [12].
Moreover, high IgG4 serum levels (> 135 mg/dl) were found in 10 out of 133 pSS patients (7.5%) in a large cohort of Greek patients, and a positive IgG4/IgG ratio (≥ 40%) was found in 3 of 6 high IgG4 serum level subjects who underwent MSGB, leading to a diagnosis of definite IgG4-RD in 3 of 133 pSS patients (2.3%) [13].
An interesting attempt to distinguish between pSS, IgG4-SA, and chronic obstructive sialadenitis was performed by Hong et al. [14] in a cohort of 155 patients. Notably, serum IgG4+ plasma cells were elevated only in the IgG4-SA group (p < 0.001), while anti-SSA/Ro was found only in one patient affected by IgG4-SA [14].
These findings highlight the differences between pSS and IgG4-SA, which, although similar, should be considered 2 distinct entities, and underline the role of anti-SSA/Ro as an exclusion criterion for IgG4-RD, as proposed in the recent ACR/EULAR classification criteria [15].
This underlines that routine research of IgG4 should not be performed in a patient suffering from xerostomia, at least in the European population. Moreover, in our opinion, immunohistochemistry should not be requested in the case of a biopsy negative for pSS: in our cohort, none of the patients with CM < 3 and/or FS < 1 was found to have an IgG4 infiltration.
Despite the lack of a large cross-sectional study, the incidence and prevalence of IgG4-RD is thought to be very low in European countries, and IgG4-SA should also be considered a very unlikely cause of xerostomia among Western populations.
The existence of an overlap syndrome “IgG4-SA/pSS” needs to be better clarified: our data did not provide evidence of any patients with such findings, but only a high number (> 20) of IgG4/HPF in 3 pSS subjects. The clinical and diagnostic meaning of this infiltrate should be better evaluated but cannot be considered enough for a diagnosis of IgG4-SA, as well as an IgG4/IgG ratio ≥ 40.
It should be stressed that tissue fibrosis with storiform pattern, obliterative vasculitis, and tissue eosinophilia remain a fundamental histological feature for the diagnosis of IgG4-SA [4]. If we only consider the presence of IgG4 in a tissue sample, we may not exclude a false positive, being IgG4 described in association to many conditions other than IgG4-RD [1].
Study limitations
Among the limits of our study were the low number of enrolled patients together with the absence of clinical data, except for the presence of xerostomia, disease duration, inflammatory markers, or organ involvement. Although we did not correlate inflammatory markers and salivary gland histopathology, it would be interesting at least to compare serological IgG4 findings with IgG4 salivary gland histopathology.
Another limitation was that no patient presented with submandibular gland swelling. This is probably related to the relatively recent onset of symptoms of the patients who were referred to our centre for MSGB but has probably underrated the incidence of IgG4-SA in our cohort. Submandibular gland swelling is classically defined as a hallmark of IgG4-SA, with patients typically presenting with subacute submandibular and/or parotid swelling [16]. A further limit could be represented by the absence of an imaging exam performed in our cohort.
In a recent paper, Sakamoto et al. [17] showed how salivary gland biopsy has low sensitivity for the diagnosis of IgG4-SA, showing favourable results with ultrasound in identifying IgG4-SA.
However, authors who focused on IgG4-RD highlighted that established features of IgG-RD according to classification criteria [18] and the presence of sialadenitis with correlation with the decreased salivary flow may direct the diagnostic procedures including histopathology assessment of MSGB towards features of IgG4-RD [16, 19].