Introduction
Cataracts caused by old age are one of the most frequent causes for blindness and poor vision worldwide. The World Health Organisation has reported that almost 50% of blindness worldwide was caused by cataracts [1]. In addition, aging, smoking, exposure to UV light, as well as genetic factors were found to significantly increase the risk of cataracts [2]. On the other hand, it remains controversial whether the use of antioxidants and alcohol could increase the risk of cataracts [2]. Several studies showed that the apoptosis of epithelial cells in the lens could result in cataractogenesis. In fact, the apoptosis of epithelial cells in the lens would suppress the growth of the lens and decrease its thickness [3]. Many researchers have observed a reduced density of epithelial cells in the lenses of patients suffering from cataracts [4–6]. Moreover, the depletion of epithelial cells from the lens could lead to impaired integrity of the fibre cells underlying the lens, thus decreasing the strength of the lens [7].
In previous studies, miR-26b was reported to play an important role in regulating lens epithelial cell growth and proliferation [8], and the deregulation of miR-26b could postpone the occurrence and development of rhegmatogenous retinal detachment via regulation of cell apoptosis and restraint of cell proliferation and invasion [9].
Until recently, non-coding RNAs (ncRNAs) were still deemed as merely playing a relatively fixed role in the cells. For example, tRNAs and rRNAs can regulate mRNA translation, while small nuclear RNAs (snRNAs) can regulate splicing. Among ncRNAs, long noncoding RNAs (lncRNAs), which belong to a class of ncRNAs of more than 200 nucleotides in length, have been shown to lack the protein-coding capacity [10, 11]. However, many studies have shown recently that lncRNAs can act as critical molecules in the pathogenesis of human malignancies [12–14]. In fact, several studies have demonstrated that lncRNAs could regulate the expression of target genes at epigenetic, posttranscriptional, and transcriptional levels [15, 16]. For example, as one of the earliest lncRNAs, discovered in 2006, myocardial infarction-associated transcript (MIAT) has been shown to affect a wide range of cellular processes and diseases, such as neurogenic commitment, nuclear body formation, paranoid schizophrenia, microvascular dysfunction, and myocardial infarction [17–22]. And a recent study also found lncRNA-MIAT to be significantly up-regulated in cataractous lenses, revealing that MIAT knockdown could affect proliferation, apoptosis, and migration of LECs via the MIAT-Akt crosstalk [23].
The family of B cell lymphoma 2 (Bcl-2) contains > 20 protein members, which can be separated into three groups depending on their structures and their roles in apoptosis [24, 25]. For example, as an anti-apoptotic protein, Bcl2 like 2 (Bcl2l2) can promote the survival of cells [25, 26]. In addition, it was shown that, by binding to BCL2L2, miR-133b could regulate the expression of BCL2L2 and subsequently mediate the apoptosis of epithelial cells in the lens [27, 28]. In fact, both the expression of miR-133b and the dysregulation of BCL2L2 have been implicated in the pathogenesis of age-induced cataracts [29]. In the above study, the authors also confirmed a negative regulatory relationship between miR-133b and BCL2L2 by conducting a computer analysis and luciferase assays.
The locus of MIAT was found on 22q12.1, a chromosomal region associated with schizophrenia [30–32]. In fact, multiple genetic mutants occurring in the genes of this chromosomal region, such as GAS2L1, RASD2, and synapsin III, have been associated with the development of schizophrenia [33–35]. It was also found that rs1894720, a single nucleotide polymorphism (SNP) in MIAT, played a significant role in the occurrence of paranoid schizophrenia. In addition, the T allele of rs1894720 has been shown to increase the risk of schizophrenia.
It has been reported that rs1894720 may alter the expression of MIAT, while MIAT may function as an endogenous competing RNA to sponge miR-26b [19]. Meanwhile, BCL2L2 is found to be a possible target of miR-26b. On the other hand, BCL2L2 acts a key regulator of epithelial cell apoptosis in the lens and is involved in the pathogenesis of cataracts [36]. Therefore, we hypothesised that rs1894720 SNP is associated with the risk of age-related cataracts by modulating the signalling pathway of MIAT/miR-26b/BCL2L2.
Material and methods
Human subjects sample collection
In this study, a total of 648 cases were enrolled from our hospital, among which 336 cases were age-related cataract patients while 312 cases were healthy controls (people without eye diseases). All experimental procedures of this study were approved by the Ethics Committee at our hospital, and all research subjects signed a form of informed consent. The enrolled cases were Chinese populations ranging from 40 to 90 years old. Clinicopathological parameters such as diabetes, hypertension, smoking, alcohol, and drugs were also investigated and included in the study (as presented in Table I). The blood samples were collected from all subjects to analyse the genotypes of their rs1894720 polymorphism in MIAT. In brief, 5 ml of sample venous blood were collected from each patient using vacuum EDTA anticoagulant blood sampling vials. The blood samples were immediately shaken 4–5 times to prevent clotting. Within 2 h of the sampling, the blood cells and plasma were separated by an ordinary centrifuge at 1500 rpm for 5 min. The separated samples were packaged in sterilised plastic tubes or freezing tubes and marked with patient names and identification numbers, and were stored at –20oC for subsequent analysis.
Table I
Demographic and clinicopathological parameters of subjects in the case and control groups in this study
Genotyping by TaqMan
The genomic DNA of blood samples was extracted and amplified by PCR. Subsequently, 2.5% agarose gel electrophoresis was used to analyse the PCR products and a restriction analysis was performed to determine the genotypes of rs1894720 SNP using a TaqMan kit (Roche, Basle, Switzerland) following the manufacturer’s instructions.
RNA isolation and real-time PCR
A miRNeasy Mini Kit (Qiagen, Hilden, Germany) was used to extract the total RNA from cell samples. Subsequently, a PrimeScript RT reagent kit (Takara, Tokyo, Japan) was used to reversely transcribe the RNA into cDNA in accordance with the instruction of the kit. The reverse transcription system was 10 μl and the reaction conditions were set as follows: three cycles of reverse transcription at 37°C for 15 min and inactivation of reverse transcriptase at 85°C for 5 s. Subsequently, the expression of MIAT, miR-26b, and BCL2L2 mRNA in the samples was measured using real-time PCR. The primers for MIAT (F: 5’-GAGATTGGCGATGGTTGTGA-3’; R: 5’-CAGTGACGCTCCTTTGTTGAA-3’), miR-26b (F: 5’-TTCAAGTAATCCAGGATAGGCT-3’; R: 5’-GAGTGTTTCAAGTAATCCAGG-3’), and BCL2L2 mRNA (F: 5’-TTCTTTGAGTTCGGTGGGGTC-3’; R: 5’-TGCATATTTGTTTGGGGCAGG-3’) were designed and synthesised by Takara (Tokyo, Japan). The real-time PCR was conducted using a SYBR® Premix Ex TaqTM II reagent kit (Takara, Tokyo, Japan) in accordance with the manufacturer’s instructions. The reaction system of real-time PCR was 20 μl, including 10.0 μl of One Step SYBR® RT-PCR Buffer III, 0.4 μl of TaKaRa Ex TaqTM HS, 0.4 μl of Prime ScriptTM RT Enzyme MixII, 0.3 μl of PCR forward: primer, 0.3 μl of PCR reverse: primer, 0.4 μl of ROX Reference Dye or DyeII (50×), 2 μl of total RNA, and 6.2 μl of RNase Free ddH2O. The reaction was carried out on an ABI 7500 quantitative PCR instrument (7500, ABI, Forest City, CA, USA) using the following reaction conditions: pre-denaturation at 95°C for 10 s; 40 cycles of denaturation at 95°C for 5 s, and annealing and final extension at 60°C for 26 s. The 2– Δ Δ Ct method was used to calculate the relative expression of target genes, with U6 (F: 5’-CTCGCTTCGGCAGCACA-3’; R: 5’-AACGCTTCA CGAATTTGCGT-3’) used as the internal reference.
Cell culture and transfection
Rabbit lens epithelial cells were isolated as described previously [34]. ARPE-19 cells and isolated rabbit lens epithelial cells were cultured in DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% foetal calf serum (FCS) (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA). The incubation conditions were 95% humidity, 5% CO2, and 37°C. To clarify the relationship between MIAT and miR-26b, the cells were seeded into six-well plates and transfected with pcDNA-MIAT, pcDNA-anti-miR-26b, MIAT siRNA, and miR-26b precursor when the cell confluence reached 30% to 50%. The transfection was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The cells were collected 48 h after transfection for subsequent measurements.
Vector construction and mutagenesis
By target gene analysis of miR-26b, we identified that MIAT and BCL2L were the potential targets of miR-26b. To clarify the relationship between MIAT and miR-26b as well as between miR-26b and BCL2L2, the segments of MIAT and BCL2L containing the binding sites for miR-26b were amplified by PCR and cloned into pcDNA vectors (Promega, Madison, WI, USA). Subsequently, site-directed mutagenesis was carried out to introduce mutations in the miR-26b binding sites of wild type MIAT and BCL2L sequences, and the mutated sequences were also amplified by PCR and cloned into pcDNA vectors. In addition, an anti-miR-26b sequence was also cloned into the pcDNA vector and amplified. The created plasmids were stored at –20°C for subsequent transfection experiments.
Luciferase assay
ARPE-19 and rabbit lens epithelial cells were seeded into six-well plates and co-transfected with MIAT/miR-26b or miR-26b/BCL2L2. After 48 h of transfection, the cells were harvested and the luciferase activity in the cells was measured using a dual-luciferase reporter gene detection kit (Promega, Madison, WI, USA) on a bioluminescence detector (Modulus, Promega, Madison, WI, USA) following the manufacturer’s instructions.
Western blot analysis
Total RNA was extracted from cell samples and mixed with a 1 × sodium dodecyl sulphate (SDS) loading buffer. A total of 20 μl loading buffer was added into each line and the proteins in the buffer were resolved by 12% polyacrylamide gel electrophoresis. After transferring the proteins onto a PVDF membrane, the membrane was blocked at room temperature for 1 h with Tris-Buffered Saline and Tween 20 (TBST) containing 5% bovine serum albumin (BSA). The membrane was then incubated with anti-BCL2L2 (Cat. no. 2724S, Cell Signaling Technology, Beverley, MA, USA) and anti- caspase-3 primary antibodies (Cat. no. 9662S, Cell Signaling Technology, Beverley, MA, USA) at 4°C overnight. On the next day, the membrane was washed by TBST and then incubated with HRP-labelled second antibodies (Cat. no. ab6728, Abcam, Cambridge, CA, USA) at room temperature for 1 h. Finally, the membrane was developed in a mixture of A and B chemiluminescence reagents (Santa Cruz Biotech, Santa Cruz, CA, USA) and visualised to analyse the relative expression of BCL2L2 and caspase-3 proteins using relative OD values.
Apoptosis analysis
The apoptosis profiles of cultured cells were measured using an Annexin V-FITC apoptosis kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instruction. The apoptosis index was calculated by measuring the apoptosis rate of sample cells using a FACSCanto II cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA).
Cell viability analysis
When the confluence of transfected cells reached ~80%, they were collected by trypsinisation, and a single cell suspension was prepared for each treatment group. Subsequently, the cells were seeded into a 96-well plate at 4 × 104 cells per well. Subsequently, 25 μl of 4 mg/ml MTT solution (Sigma-Aldrich, St. Louis, MO, USA) were added into each well. After being incubated for 4 h in an incubator, 150 μl of dimethyl sulphoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) were added into each well and gently mixed for 10 min in a shaker. The OD value in each well was measured on a Modulus plate reader at 24 h, 48 h, and 72 h time points, respectively, using a wavelength of 490 nm. Subsequently, incubation time was used as the x-axis while OD values were used as the y-axis to draw a standard curve and calculate the cell viability. Each experiment was repeated three times.
Statistical analysis
Data analysis was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The measurement data was expressed as mean and standard deviation (SD). The comparison between two groups was carried out using t tests, while the comparison among several groups was carried out using logistic regression and one-factor ANOVA. The count data was expressed as percentage and rate, and it was verified using the χ2 test. A p-value of < 0.05 was considered as statistically significant.
Results
Rs1894720 polymorphism was associated with the risk of age-related cataract
We investigated the correlation between rs1894720 polymorphism in MIAT and the incidence of age-related cataract. As shown in Table I, there were 336 age-related cataract subjects and 312 healthy controls recruited to this study. In addition, the patients of age-related cataract were genotyped as GG (n = 132), GT (n = 150), and TT (n = 30). Although no significant differences were observed in terms of their characteristics between the patient group and the control group, the OR and p-values of the patients genotyped as GT and TT indicated that they were associated with a higher risk of age-related cataracts.
Myocardial infarction-associated transcript functioned as an endogenous competing RNA to sponge miR-26b
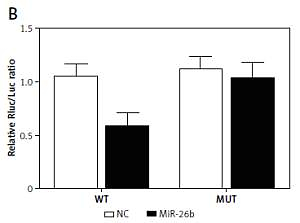
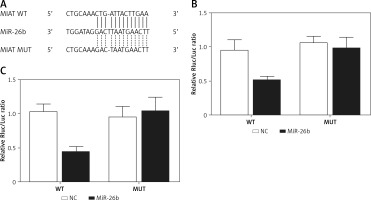
Using bioinformatics tools, we identified a putative binding site of miR-26b in the 3’UTR of MIAT (Figure 1 A). To clarify the regulatory relationship between MIAT and miR-26b, luciferase assays were conducted by co-transfecting ARPE-19 and rabbit lens epithelial cells with miR-26b and wild-type/mutant MIAT plasmids. As demonstrated in Figure 1, the luciferase activity of ARPE-19 (Figure 1 B) and rabbit lens epithelial cells (Figure 1 C) co-transfected with miR-26b and wild-type MIAT was significantly reduced compared to the luciferase activity in other groups. Therefore, MIAT may function as an endogenous competing RNA to sponge miR-26b.
Figure 1
Myocardial infarction-associated transcript (MIAT) functioned as an endogenous competing RNA to sponge miR-26b. A – Putative binding site of miR-26b was found in the 3’UTR of MIAT. B – Reduced luciferase activity in ARPE-19 cells transfected with miR-26b and wild-type MIAT. C – Reduced luciferase activity in rabbit lens epithelial cells transfected with miR-26b and wild-type MIAT
MiR-26b negatively regulated the expression of BLC2L2 via targeting BLC2L2
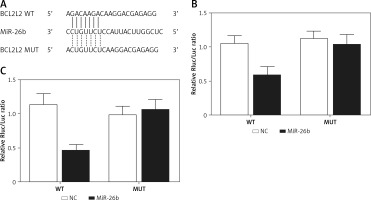
BCL2L2 has been identified as a possible target of miR-26b and contains a binding site for miR-26b (Figure 2 A). Therefore, PETNCE01 and PTECE01 cells were co-transfected with miR-26b and wild-type or mutant BLC2L2. As shown in Figure 2 B, the luciferase activity of PETNCE01 and PTECE01 cells co-transfected with miR-26b and wild-type BLC2L2 was evidently inhibited compared with that in other transfection groups, thus verifying the role of BCL2L2 as a virtual target gene of miR-26b.
Figure 2
BCL2L2 was a virtual target gene of miR-26b. A – BCL2L2 was identified as a target gene of miR-26b and contained a specific binding site for miR-26b. B – Reduced luciferase activity in ARPE-19 cells transfected with miR-26b and wild-type BCL2L2. C – Reduced luciferase activity in rabbit lens epithelial cells transfected with miR-26b and wild-type BCL2L2
Myocardial infarction-associated transcript elevated the expression of BCL2L2 via inhibition of the expression of miR-26b
ARPE-19 cells were transfected with MIAT, anti-miR-26b, or negative controls. As shown in Figure 3, the relative expression of miR-26b (Figure 3 A) was obviously suppressed in ARPE-19 cells transfected with MIAT or anti-miR-26b. In contrast, the relative expression of BCL2L2 mRNA (Figure 3 B) was apparently up-regulated with the transfection of MIAT or anti-miR-26b, and these results were further validated by western blot, which showed clearly visible protein bands of BCL2L2 (Figure 3 C) and an elevated relative protein density of BCL2L2 (Figure 3 D). Similar results were also observed in rabbit lens epithelial cells (Figures 3 E–H), thus validating the positive effect of MIAT on the expression BCL2L2 via inhibition of the expression of miR-26b. Therefore, a MIAT/miR-26b/BCL2L2 signalling pathway could be established accordingly.
Figure 3
Myocardial infarction-associated transcript (MIAT) inhibited the expression of miR-26b but elevated the expression of BCL2L2. A – Relative expression of miR-26b was inhibited in ARPE-19 cells transfected with MIAT or anti-miR-26b. B – Relative expression of BCL2L2 mRNA was up-regulated in ARPE-19 cells transfected with MIAT or anti-miR-26b. C – Protein band of BCL2L2 was clearly visible but the protein band of caspase-3 was negligible in ARPE-19 cells transfected with MIAT or anti-miR-26b. D – Relative density of BL2L2 protein was up-regulated in ARPE-19 cells transfected with MIAT or anti-miR-26b. E – Relative expression of miR-26b was inhibited in rabbit lens epithelial cells transfected with MIAT or anti-miR-26b. F – Relative expression of BCL2L2 mRNA was up-regulated in rabbit lens epithelial cells transfected with MIAT or anti-miR-26b. G – Protein band of BCL2L2 was clearly visible, but the protein band of caspase-3 was negligible in rabbit lens epithelial cells transfected with MIAT or anti-miR-26b. H – Relative density of BL2L2 protein was up-regulated in rabbit lens epithelial cells transfected with MIAT or anti-miR-26b

Myocardial infarction-associated transcript suppressed inflammatory responses and cell apoptosis via inhibition of the expression of miR-26b
Subsequently, in order to identify the regulatory effect of BCL2L2 on the apoptosis of epithelial cells in the lens, the expression of caspase-3 was measured in ARPE-19 and rabbit lens epithelial cells treated by MIAT, anti-miR-26b, or negative controls. The transfection of ARPE-19 and rabbit lens epithelial cells with MIAT and anti-miR-26b evidently decreased the protein band intensity (Figures 3 C, G) and relative density (Figures 4 A, B) of caspase-3. Moreover, we also measured the apoptosis index and cell viability in transfected ARPE-19 and PTENCE-2 cells. As shown in Figure 4, a lower apoptosis index was obtained in the ARPE-19 (Figure 4 C) and rabbit lens epithelial cells (Figure 4 E) transfected with MIAT or anti-miR-26b, accompanied by higher cell viability (Figures 4 D, F). Therefore, MIAT could suppress cell apoptosis via inhibiting miR-26b expression.
Figure 4
Myocardial infarction-associated transcript (MIAT) suppressed inflammatory responses and cell apoptosis. A – Relative density of caspase-3 protein was down-regulated in ARPE-19 cells transfected with MIAT or anti-miR-26b. B – Relative density of caspase-3 protein was down-regulated in rabbit lens epithelial cells transfected with MIAT or anti-miR-26b. C – Apoptosis index of ARPE-19 cells was reduced when they were transfected with MIAT or anti-miR-26b. D – Higher cell viability was observed in ARPE-19 cells transfected with MIAT or anti-miR-26b. E – Apoptosis index of rabbit lens epithelial cells was reduced when they were transfected with MIAT or anti-miR-26b. F – Higher cell viability was observed in rabbit lens epithelial cells transfected with MIAT or anti-miR-26b
Myocardial infarction-associated transcript was involved in the pathogenesis of age-related cataract
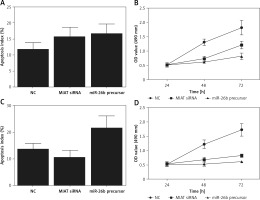
To further validate the role of MIAT/miR-26b/BCL2L2 signalling pathway, ARPE-19 cells were transfected with MIAT siRNA, miR-26b precursor, or negative controls. As shown in Figure 5, the relative expression of MIAT (Figure 5 A), BCL2L2 mRNA (Figure 5 C), and BCL2L2 protein (Figures 5 D, E) was obviously reduced in the presence of MIAT siRNA or miR-26b precursor, whereas the relative expression of miR-26b (Figure 5 B) and caspase-3 (Figures 5 E, F) was evidently up-regulated in the presence of MIAT siRNA or miR-26b precursor. Similar results were obtained when the above experiments were repeated in rabbit lens epithelial cells (Figures 5 G–L), suggesting the presence of a MIAT/miR-26b/BCL2L2 signalling pathway. In addition, the apoptosis index was up-regulated in ARPE-19 (Figure 6 A) and rabbit lens epithelial (Figure 6 C) cells transfected with MIAT siRNA or miR-26b precursor, accompanied by a reduced level of cell viability (Figures 6 B, D). Therefore, it could be concluded that MIAT was involved in the pathogenesis of age-related cataract via modulating inflammatory responses and cell viability. Moreover, since rs1894720 polymorphism (genotype TT) could down-regulate the expression of MIAT, leading to reduced BCL2L2 expression by increasing the expression of miR-26b, the apoptosis of epithelial cells in the lens would be enhanced accordingly, thus increasing the incidence of age-related cataract.
Figure 5
Myocardial infarction-associated transcript (MIAT) was involved in the pathogenesis of age-related cataract via regulating the expression of miR-26b and its downstream signalling pathway. A – Relative expression of MIAT was reduced in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor. B – Relative expression of miR-26b was elevated in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor. C – Relative expression of BCL2L2 mRNA was reduced in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor. D – Protein band of BCL2L2 protein was negligible but the protein band of caspase-3 was thicker in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor. E – Relative density of BCL2L2 protein was reduced in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor. F – Relative density of caspase-3 was elevated in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor G – Relative expression of MIAT was reduced in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor. H – Relative expression of miR-26b was elevated in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor. I – Relative expression of BCL2L2 mRNA was reduced in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor. J – Protein band of BCL2L2 protein was negligible but the protein band of caspase-3 was thicker in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor. K – Relative density of BCL2L2 protein was reduced in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor. L – Relative density of caspase-3 was elevated in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor

Figure 6
Myocardial infarction-associated transcript (MIAT) suppressed inflammatory responses and cell apoptosis. A – Apoptosis index of ARPE-19 cells was increased when they were transfected with MIAT siRNA or miR-26b precursor. B – Suppressed cell viability was observed in ARPE-19 cells transfected with MIAT siRNA or miR-26b precursor. C – Apoptosis index of rabbit lens epithelial cells was increased when they were transfected with MIAT siRNA or miR-26b precursor. D – Suppressed cell viability was observed in rabbit lens epithelial cells transfected with MIAT siRNA or miR-26b precursor
Discussion
In this study, the OR and P values of the patients genotyped as GT and TT in their rs1894720 SNP of MIAT indicated that they were associated with a higher risk of age-related cataracts. Therefore, the rs1894720 SNP (genotype TT) could down-regulate the expression of MIAT, thus leading to reduced BCL2L2 expression by increasing the expression of miR-26b. As a result, the apoptosis of epithelial cells in the lens would be enhanced, thus increasing the incidence of age-related cataract. It was shown that the cataracts in humans were caused by the apoptosis of a large portion of epithelial cells in the lens, suggesting that apoptosis can induce the formation of cataracts. Some researchers have used in vitro experiments to show that the apoptosis of epithelial cells in the lens could be induced by oxidative stress. Furthermore, it was demonstrated that the apoptosis of epithelial cells in the lens led to both spatial and temporal opacification, indicating the critical role played by epithelial cell apoptosis during the formation of cataracts.
Bcl2l2 is a member of the Bcl-2 protein family. As an anti-apoptotic regulator, Bcl2l2 can promote cell survival and decrease cell apoptosis under cytotoxic conditions [37, 38]. Bcl2l2 is also aberrantly expressed in a wide range of cancer cells and has been implicated in carcinogenesis [39]. It was reported that BCL2L2 could be targeted and suppressed by miR-133b. Therefore, the collective functions of BCL2L2 and miR-133b would determine the apoptosis and viability of the epithelial cells in the lens, thus contributing to the pathogenesis of cataracts. In this study, BCL2L2 was identified as a target of miR-26b and contained a specific binding site for miR-26b. Luciferase activity was reduced in cells co-transfected with miR-26b and wild-type BLC2L2 compared with that in the negative controls, thus verifying the role of BCL2L2 as a virtual target gene of miR-26b.
The miR-26 family contains two mature miRNAs, i.e. miR-26b and miR-26a. These two miRNAs have both been reported to act as tumour suppressors in the development of glioma, breast cancer, and hepatocellular carcinoma [40–43]. In addition, miR-26b could inhibit the growth of tumour cells while inducing their apoptosis by regulating the expression of SLC7A11 and PTGS2 [40, 41]. Furthermore, miR-26b was confirmed to negatively regulate the proliferation and metastasis of LECs, indicating that miR-26b may play a role in LEC lesions [44, 45]. To sum up, the downregulation of miR-26b could delay the progression of oxidative cataracts by inhibiting NF-κB expression, suppressing inflammatory factors, restraining the apoptosis of LECs, and regulating cell proliferation. Therefore, miR-26b may be selected as a target for cataract prevention and treatment.
In this study, we validated that miR-26b and MIAT could interact with each other as endogenous competing RNAs. MIAT was initially found to be over-expressed in post-mitotic precursor cells in the retina [46]. MIAT is highly conserved in many mammalian species. In addition, MIAT has emerged recently as an important regulator of diabetic complications [47]. Furthermore, the level of MIAT was significantly increased in the anterior chamber (AH) of patients with cataracts. In contrast, the level of MIAT in the AH harvested from patients of other ocular diseases showed no increase. It was found that the increased level of local MIAT in the lens is caused by diseased epithelial cells. In fact, the presence of MIAT in AH suggested that MIAT might participate in the degeneration of tissues lining the AH. In addition, an increased level of MIAT may change the function of gene regulatory networks involved in cataract formation. Apart from MIAT, many lncRNAs were reported to play an active role in many pathogeneses of human diseases. For example, lncRNA PVT1 was once reported to represent a potential therapeutic target for the treatment of multiple myeloma because it could promote multiple myeloma cell proliferation and induce cell apoptosis by inhibiting its target gene, miR-203a [48]. And lncRNA, UBE2CP3-001, was found to be potentially applied in the anti-glioma therapy [49].
In this study, caspase-3 expression was evidently decreased in cells transfected with MIAT or anti-miR-26b, accompanied by a reduced apoptosis index and higher cell viability, indicating that MIAT could suppress cell apoptosis via inhibiting miR-26b expression. In line with these results, we found that the relative expression of MIAT and BCL2L2 was obviously reduced in the presence of MIAT siRNA or miR-26b precursor, whereas the relative expression of miR-26b and caspase-3 was evidently up-regulated in the presence of MIAT siRNA or miR-26b precursor. Furthermore, the apoptosis index was increased while the cell viability was decreased in cells transfected with MIAT siRNA or miR-26b precursor. Cataract patients usually show accumulated levels of oxidative stress products. Therefore, the higher expression of MIAT in cataract patients is consistent with the higher level of oxidative stress observed in these patients. A high level of oxidative stress decreases the growth and viability of HLECs, whereas the knockdown of MIAT expression further decreases the growth and viability of HLECs, suggesting that an increased level of MIAT may help to combat oxidative stress. In cataract patients, the level of MIAT was increased in their AH, lens, and plasma. In addition, an in vitro study was carried out to demonstrate the essential role of MIAT in the regulation of HLEC migration and proliferation. The authors found that the knockdown of MIAT inhibited TNF-α-induced migration and proliferation of HLECs, indicating that MIAT intervention may affect the formation of PCO.
It was found that the SNP rs1894720 in MIAT was significantly correlated with the onset of paranoid schizophrenia, while the T allele of rs1894720 could increase the risk of paranoid schizophrenia. In this study, we identified the association between rs1894720 SNP and the risk of age-related cataract. This observation was consistent with the regulatory association among MIAT, miR-26b, and BCL2L2. We also postulated that the presence of rs1894720 SNP in the epithelial cells of the lens could alter the expression level of MIAT, thus leading to changed expression of miR-26b and BCL2L2. As a result, the apoptosis profile of epithelial cells could be altered as well, thus resulting in elevated risk of cataract. There are limitations of this study: The cases of the present study were collected from a Chinese population instead of a more diverse population, leading to a potential limitation of the genotype-based analysis of the association between rs1894720 polymorphism and age-related cataract. Also, the MIAT/miR-26b/BCL2L2 signalling pathway was mainly established in the cellular model, while it would be better verified in an in vivo model if the clinical implications are to be discussed. Moreover, a larger sample size is required in further studies to confirm the results of this study.
The findings of this study presented higher incidence of age-related cataract upon the presence of rs1894729 SNP (G>T), which contributed to the possible implication that rs1894729 SNP would be used as an indicator to predict the risk of age-related cataract. Meanwhile, our study also established the MIAT/miR-26b/BCL2L2 signalling pathway, thus providing a potential therapeutic modality for the disease control of age-related cataract.
In conclusion, the results of the present study showed that rs1894720 SNP may alter the expression of MIAT. Meanwhile, BCL2L2 is a possible target of miR-26b and a key regulator of epithelial cell apoptosis in the lens and is hence implicated in the pathogenesis of cataracts. We hypothesised that the rs1894720 SNP is associated with the risk of age-related cataract by modulating the signalling pathway of MIAT/miR-26b/BCL2L2.