Introduction
Congenital obstructive nephropathy (CON) results from multiple functional and morphological disorders associated with obstruction in the urine flow, which are responsible for interstitial fibrosis, tubular and vascular atrophy, and glomerular sclerosis. Glomerular hyperfiltration and glomerular sclerosis of the remaining nephrons lead to chronic kidney disease (CKD) [1]. Although congenital obstruction of the urinary tract is not always related to CON [2], the unfavorable prognosis in children with obstructive uropathy mandates a search for new, simple markers, which would enable early diagnosis of renal injury and early intervention in children at risk of renal damage, and predict long-term outcome.
Periostin is an extracellular matrix (ECM) protein, induced during embryogenesis in tissues exposed to mechanical load such as periodontal ligament, periosteum, cardiac valves, annuli, lung, stomach, skin, and tendons [3], and detected only in small amounts in healthy people [4]. Periostin is produced de novo in kidneys, heart, lung, skin, bone, in allergic and metabolic chronic diseases, and during mechanical stress and cancer growth. It promotes the progression of fibrosis via ECM remodeling and inflammatory cell infiltration, and is involved in post-injury tissue repair and wound healing [3, 5]. Cytokeratin-18 (CK-18) is a cytoskeletal protein widely expressed in epithelial and parenchymal cells. It plays a pivotal role in maintaining cell shape, integrity, cell signaling, intracellular organization, transport, differentiation, and proliferation. It protects the cell from mechanical and non-mechanical stress [6]. CK-18 has been investigated in experimental and clinical studies, and found to be a potential marker of various pathological conditions such as heart failure, liver diseases, cancers, acute kidney injury (AKI) and CKD [5, 7, 8].
To the best of our knowledge, there are no studies in the available literature investigating the usefulness of serum periostin and CK-18 in humans with CON. The aim of this study was to assess serum and urine periostin and CK-18 in children with CON in relation to CON etiology, treatment, and kidney injury.
Material and methods
This cross-sectional single-center study was performed in 81 children with CON, who were treated in a tertiary pediatric nephrology center in Poland. The study was conducted between July 2016 and April 2019. The inclusion criteria were: age from 6 months to 18 years and presence of CON secondary to ureteropelvic junction obstruction (UPJO), ureterovesical junction obstruction (UVJO), and posterior urethral valves (PUV). The exclusion criteria were: the presence of chronic allergic, inflammatory, or fibrotic disease, coexisting anomalies of the urinary tract, except secondary vesicoureteral reflux (VUR) in children with PUV, surgical intervention in the last 12 months, urinary tract infection (UTI) in the last 6 months and acute infection (temporary exclusion). The control group consisted of 60 healthy children with normal results of blood and urine tests, and ultrasound (US) of the urinary tract, and no history of allergy. The flow diagram of the participants is displayed in Figure 1.
In all participants, age (years), sex, serum, and urine creatinine (Cr) concentration were evaluated. Estimated glomerular filtration rate (GFR; ml/min/1.73 m2) according to the revised 2009 Schwartz formula was calculated [9]. Urinalysis and urine culture were performed to rule out UTI. Biochemical parameters were measured using the VITROS 5600 Integrated System (Ortho Clinical Diagnostics). The normal value of serum Cr (sCr) depending on age was 0.2-0.7 mg/dl. Blood and urine samples were obtained on fasting in the morning, immediately centrifuged according to the manufacturers’ instructions, frozen, and stored at –80°C. The biomarkers were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits. Serum periostin (sPeriostin) was assessed using kit Cat. No. BI 20433 from Biomedica Medizinprodukte GmbH, Vienna, Austria; urine periostin (uPeriostin) was assessed using kit Cat. No. HUDLO 02367 Human Periostin (POSTN) from Reagent Genie, Dublin, Ireland; serum CK-18 (sCK-18) and urine CK-18 (uCK-18) were assessed using kit Cat. No. HUFI 02320, Human CK-18/KRT18/Cytokeratin-18 from Reagent Genie, Dublin, Ireland. Urine biomarkers were adjusted to the urine Cr (uCr) levels, measured from the same urine samples, and expressed as nanograms per milligram Cr (ng/mg).
In all children, abdominal US and 99mTechnetium-ethylenedicysteine (99mTc-EC) renal scintigraphy (SC) were performed at study entry. US was performed using a Philips Epiq 5G device (Royal Philips, Amsterdam, The Netherlands) in B-mode. Pelvic dilatation was classified based on the anterior-posterior diameter (APD) of the renal pelvis as follows: mild APD < 15 mm, moderate APD 15-25 mm, severe APD > 25 mm [10]. Renal SC was conducted to assess differential renal function (DRF) of the affected kidney, half-time (T1/2) of tracer excretion, and permanent lesions in renal parenchyma. DRF < 40% and > 55% was considered abnormal and T1/2 > 20 min suggested obstruction [11]. CON was diagnosed based on the presence of permanent lesions in renal parenchyma: narrowed parenchymal layer or abnormal renal outline, and disorder in tracer uptake. Voiding cystourethrography was performed at the time of uropathy diagnosis to rule out VUR.
The study was approved by the local Bioethics Committee (permission No. KB/152/2016) before initiation and was conducted in accordance with the guidelines of the 2013 Declaration of Helsinki. Written informed consent was obtained from all participant representatives and participants (≥ 16 years) prior to study inclusion.
Statistical analysis
Statistical analysis was performed using the program Statistica ver. 13.3 PL (StatSoft, Tulsa, OK, USA). Data distributions and tests of normality were evaluated with the Shapiro-Wilk test. Data were reported as absolute numbers and percentages, mean ± standard deviation or median and interquartile ranges (IQRs). Student’s t-test or the Mann-Whitney U-test (depending on variables distribution) was used to compare two groups of variables. Differences between four subgroups were analyzed using the ANOVA or Kruskal-Wallis test with a post hoc test. Pearson’s or Spearman’s rank correlation evaluated the correlations between variables. Receiver operating curve (ROC) analysis was performed to determine the best cut-off, sensitivity, specificity, and accuracy of serum and urine periostin and CK-18 for detection of CON. P values less than 0.05 were considered statistically significant for all tests.
Results
We evaluated 81 children with CON secondary to UPJO (65%), UVJO (21%) and PUV (14%), and 60 healthy children as a control group (Table 1). CON was diagnosed more often in boys than girls. Defect of the urinary tract was recognized the earliest in children with UVJO. These children were also the youngest at the study entry and at the time of surgery. APD of renal pelvis > 25 mm in US was found only in 19% of patients with UPJO. Reduced DRF of affected kidney < 40% in SC was found in 24% of patients, including 25% of patients with UPJO, 12% with UVJO, and 36% with PUV. According to T1/2 tracer excretion > 20 min, significant obstruction was found in 52% of patients, including 62% of patients with UPJO, 35% with UVJO, and 27% with PUV. Conservative treatment was used in 59% of children, 24% of children were included in the study before surgery, and 17% after surgery. 18 (34%) children with UPJO and 4 (24%) with UVJO required surgical treatment. In children with PUV, valve resection was performed.
Table 1
Demographic and clinical data of the study groups and the control group
Children with UPJO had significantly lower mean sPeriostin levels than the controls, and children with UPJO, UVJO, and PUV had significantly higher median uPeriostin and uPeriostin/Cr levels than the controls (Table 2). Median sCK-18 and uCK-18/Cr levels were significantly higher in UVJO patients, and mean uCK-18 level was significantly higher in PUV patients than the controls. Children with UPJO and PUV showed significantly lower mean GFR levels than the controls (106.15 ±16.27, 96.51 ±16.56 vs. 121.60 ±26.09, p < 0.001, p = 0.002 respectively). The post hoc test confirmed significantly lower levels of sPeriostin in children with UPJO, significantly higher levels of uPeriostin in children with UPJO, UVJO, and PUV, and significantly higher levels of uPeriostin/Cr in UPJO and UVJO children than in the controls (Fig. 2). There were no significant differences in serum and urine periostin and CK-18 levels between children with UPJO, UVJO and PUV. Children with UPJO and PUV had significantly higher median Cr levels than UVJO patients (0.40, 0.30-0.50; 0.50, 0.30-0.70 vs. 0.30, 0.20-0.30; p = 0.002, p = 0.005 respectively) and children with PUV demonstrated significantly lower mean GFR levels than UVJO patients (96.51 ±16.56 vs. 115.90 ±23.05, p = 0.035). No other differences between Cr and GFR levels and CON etiology were found.
Fig. 2
Comparison of biomarkers in children with UPJO, UVJO, PUV, and the controls: A) serum periostin, B) urine periostin, C) urine periostin/creatinine ratio
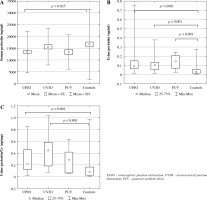
Table 2
Comparison of biomarkers between children with different etiology of CON and the controls. A. Comparison of biomarkers in children with UPJO and the controls. B. Comparison of biomarkers in children with UVJO and the controls. C. Comparison of biomarkers in children with PUV and the controls
Children conservatively treated and those before and after surgery had significantly higher median uPeriostin and uPeriostin/Cr levels than the controls (Table 3). However, those after surgery had a significantly lower mean sPeriostin level than the controls. There were no other differences in biomarker levels between studied groups and the controls. The post hoc test confirmed significantly higher uPeriostin levels in all patients, regardless of CON treatment and significantly higher uPeriostin/Cr levels in children conservatively treated and before surgery compared to the controls (Fig. 3).
Fig. 3
Comparison of biomarkers in children with a different type of CON treatment and the controls A) urine periostin, B) urine periostin/Cr ratio
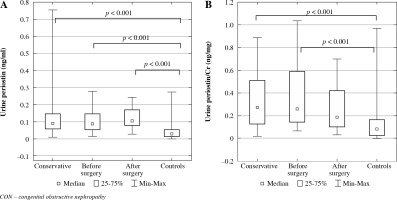
Table 3
Comparison of biomarkers between children with different types of CON treatment and the controls. A. Comparison of biomarkers between children treated conservatively and the controls. B. Comparison of biomarkers between children before surgery and the controls. C. Comparison of biomarkers between children after surgery and the controls
We found no differences in serum and urine biomarkers depending on APD of the renal pelvis in US, and DRF values in SC, as well as no correlations between biomarkers and GFR and APD (data not shown). We only found a strong negative correlation of uPeriostin with DRF < 40% (r = –0.53, p = 0.020).
ROC analysis demonstrated the highest usefulness of uPeriostin area under the curve (AUC: 0.831) and uPeriostin/Cr (AUC: 0.768), and low usefulness of sPeriostin (AUC: 0.656) and uCK-18 (AUC: 0.615) for detecting renal injury in children with CON (Table 4, Fig. 4). sCK-18 showed the highest specificity and uCK-18 the highest sensitivity for detecting renal injury in those children.
Fig. 4
Diagnostic usefulness of biomarkers for detecting renal injury in children with obstructive nephropathy A) serum periostin (ng/ml), B) urine periostin (ng/ml), C) urine periostin/Cr (ng/mg), D) urine cytokeratin-18 (ng/ml)
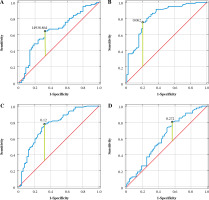
Table 4
Diagnostic usefulness of biomarkers for detecting renal injury in children with obstructive nephropathy
The study demonstrated mutual correlations between biomarkers. sPeriostin showed positive correlations with uPeriostin/Cr and uCK-18/Cr in the study group, and uPeriostin in the control group (Table 5). sCK-18 showed positive correlations with uCK-18 and uCK-18/Cr in the study group, and uCK-18/Cr in the control group.
Table 5
Correlations of serum periostin and cytokeratin-18 with other biomarkers in the study group and the control group
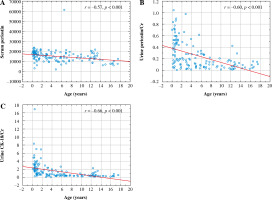
Serum and urine biomarkers demonstrated negative correlations with age. sPeriostin, uPeriostin/Cr and uCK-18/Cr revealed strong negative correlations with age in the study group (Fig. 5), and uPeriostin/Cr, sCK-18, and uCK-18/Cr showed weak negative correlations with age in the control group (r = –0.32, p = 0.014; r = –0.32, p = 0.011; r = –0.50, p < 0.001, respectively). We also found strong negative correlations of uPeriostin/Cr and uCK-18/Cr with uCr in the study group (r = –0.68, r = –0.93, respectively, p for all < 0.001) and the control group (r = –0.59, r = –0.68, respectively, p for all < 0.001).
Discussion
To date, more than 100 studies have been published evaluating the usefulness of biomarkers in CON. The authors mainly studied patients with unilateral ureter obstruction (UUO) [12, 13]. We also evaluated children with CON secondary to UVJO and PUV, and found that CON was more often diagnosed in boys than girls, which is in line with other studies [11, 14]. Our study demonstrated that children with different CON etiology showed higher uPeriostin and uPeriostin/Cr levels than the controls. Although children with PUV have the worst long-term prognosis [15], we found no differences in biomarker levels between children with UPJO, UVJO, and PUV. It could be related to the small number of PUV children in our cohort. Periostin has been proposed as a marker of CKD. It is induced de novo in tubular injury and interstitial renal fibrosis. Increased expression of uPeriostin was reported in several animal models of kidney injury such as UUO, 5/6 nephrectomy, ischemic/reperfusion, and in patients with AKI and CKD due to progressive glomerulonephritis, diabetic and chronic allograft nephropathy (CAN) and polycystic kidney disease [4, 16-18].
Unexpectedly, patients with UPJO were found to have lower levels of sPeriostin than the controls. Similarly, in the study by Fujitani et al. sPeriostin levels were significantly higher in the control group than in the clinical and subclinical allergy group until age 3 [19]. When interpreting sPeriostin levels, it should be remembered that periostin is a relatively nonspecific biomarker and may be influenced by different factors, such as allergy or heart disease [3, 20]. Moreover, sPeriostin is of limited usefulness in children due to high secretion by osteoblasts, bone turnover, and growth in this age [20]. The heterogeneity of patient age in our study may have influenced the results. We also found that children with UVJO showed significantly higher levels of sCK-18 and uCK-18/Cr, and children with PUV had a higher level of uCK-18 than the controls. CK-18 is released into the circulation in response to cell death [8]. The presence of CK-18 in urine is most likely related to the progressive degradation of tubular cells. An increase of uCK-18 levels was found in animal models of adenine nephropathy, Alport syndrome, and patients with AKI and CKD due to different types of renal disease [6, 21].
The US and SC results do not always allow to identify patients who need surgical treatment to prevent renal injury and those who may be safely observed [2, 11]. In our study, 24% of patients were included in the study before surgery, and 17% of patients after surgery. Therefore, we could not assess the usefulness of US, SC and biomarkers to distinguish between children who need surgical treatment and those who may be conservatively treated. We found no relations between biomarkers and results of US and SC, except a strong negative correlation between uPeriostin and decreased DRF < 40%. This finding is consistent with previous studies testing the usefulness of biomarkers in UUO [22, 23]. Paraboschi et al., based on a systematic review in 2019, assessed 28 biomarkers in terms of indications for surgical treatment in UUO and concluded that, due to low sensitivity or specificity, none of the biomarkers could be validated in routine practice [13]. In the present study, we found no differences in serum and urine periostin and CK-18 between children conservatively treated and those before and after surgery. However, regardless of treatment method, children with CON showed significantly higher uPeriostin and uPeriostin/Cr levels than the controls. All children with CON showed lower levels of sPeriostin than the controls, but the difference was significant only in children after surgery.
Some researchers noted that high preoperative levels of urine biomarkers decreased significantly 2 to 12 months after surgery [13]. In contrast, the results of others studies have indicated elevated levels of biomarkers despite correction of obstruction due to ongoing renal injury [24, 25]. In our study, elevated uPeriostin levels are indicative of progressive renal injury. The experimental model of UUO demonstrated high levels of uCK-18 in the early and advanced stages of the disease [6]. In contrast, Roth et al. reported elevated levels of sCK-18 and uCK18 only in the advanced stages of CKD [21]. It is possible that the lack of difference in CK-18 levels in our cohort could be attributed to normal sCr levels in the majority of our children, as well as to the diverse mechanism of action of these markers. Periostin plays a central role in renal damage and remodeling in CKD, while CK-18 is a cytoprotective protein [6].
Currently used imaging techniques do not provide information about the early stage of renal injury. Conventional laboratory parameters increase only after significant loss of functioning nephrons [1]. Although all our patients demonstrated varying intensity of renal injury in SC in this study, and 24% of them have DRF less than 40%, slightly elevated sCr was seen only in 7.4% of patients. ROC analysis demonstrated higher usefulness of uPeriostin than uPeriostin/Cr and sPeriostin for detecting renal injury in children with obstructive uropathy. Among the studied biomarkers, uPeriostin had the highest area under the curve (AUC: 0.831) and accuracy (77.3%). The results of our study revealed some similarities with other studies. Satirapoj et al. reported a good diagnostic profile of uPeriostin/Cr (AUC: 0.830) for detecting renal fibrosis in CAN patients [17]. Hwang et al. reported a moderate diagnostic profile of uPeriostin/Cr (AUC: 0.782) for predicting CKD progression in IgA nephropathy [18]. We found low usefulness of uCK-18 (AUC: 0.613) for detecting renal injury. However, sCK-18 showed the highest specificity (98.3%) and uCK-18 the highest sensitivity (80.2%) for detecting renal injury in obstructive nephropathy. Roth et al. showed the potential of serum and urine CK-18 for predicting advanced stages of CKD [21], and Schmitt et al. pointed out the potential of sCK-18 for predicting delayed renal graft function [26]. Our previous study demonstrated a high diagnostic profile of uPeriostin (AUC: 0.849) and low profile of uCK-18 for diagnosing advanced renal scars in children with CON. However, uPeriostin did not differentiate advanced scars from borderline lesions [27].
This study revealed mutual correlations between biomarkers. We can speculate that the positive sPeriostin relation with uPeriostin could be associated with periostin glomerular filtration [4], and a positive correlation of sCK-18 with uCK-18 may be the result of renal reabsorption due to the low molecular weight of CK-18. This hypothesis is supported by the results of Roth et al., who reported higher levels of sCK-18, and increased fractional excretion of uCK-18 in advanced stages of CKD [21].
In our study, serum biomarkers and urine biomarkers normalized to Cr demonstrated negative correlations with age. Limited studies have confirmed a negative correlation of sPeriostin with age [20, 28, 29]. Reported data on sCK-18 are inconclusive. No age dependence of sCK-18 and uCK-18 has been demonstrated in the adult population [21, 30]. In the pediatric population, available studies show a negative correlation of the serum caspase-cleaved fragments of CK-18 with age [31]. These fragments are considered as markers of increased apoptosis during CKD progression [21]. In the current study, the negative correlation of normalized biomarkers with age could be influenced by the relatively large number of young children. Low urine Cr excretion in young children results in higher values of normalized biomarkers than in older ones [1]. We found no relations of uPeriostin and uCK-18 with age in the study group. That is why we can assume that these two molecules reflect a pathological process of kidney damage in children with CON. However, more research is needed to evaluate their exact role in kidney disease.
This study is limited by its cross-sectional nature, preventing us from drawing a causal conclusion. A single-center study design may result in bias occurrence. Heterogenicity of patient ages, difference in treatment method and patients’ follow-up as well as relatively small subgroups may have influenced the results.
Conclusions
Neither biomarker demonstrated any relation with the etiology of CON. However, all patients, regardless of etiology of CON, demonstrated higher levels of uPeriostin and uPeriostin/Cr than the controls, UVJO patients showed higher levels of sCK-18 and uCK-18, and PUV patients showed higher levels of uCK-18/Cr than the controls. We also found no relation with CON treatment. Regardless of the type of treatment, patients revealed higher levels of uPeriostin and uPeriostin/Cr than the controls. We tested a panel of biomarkers, but only uPeriostin seems to be a useful tool for detecting renal injury in children with CON, especially due to its strong negative correlation with DRF < 40%. We believe that the evaluation of uPeriostin may help the management of CON in children.