Introduction
Acute appendicitis (AA) is one of the most common emergencies [1–3] and is the reason for 10–20% of all acute surgical admissions for abdominal pain [4]. Likewise, appendectomies remain among the most frequently performed emergency surgical procedures [1, 5]. Its lifetime risk and incidence are estimated to be 7–8% [1, 6] and 88/100 000 per year, respectively [2]. Despite the high prevalence, this condition is commonly misdiagnosed [7], with a negative appendectomy rate as high as 10–40% according to previous studies [8, 9].
The lack of reliable and unambiguous markers for AA is cited as the main reason for the false-positive diagnoses [1, 5]. Therefore, the diagnostic approach is still based on the clinical presentation and laboratory tests (white blood cells, C-reactive protein), which have limited accuracy even if combined with scoring systems (such as the Alvarado score) [1, 5, 10–12]. Lately, the sensitivity improved due to the introduction of imaging studies, such as ultrasound and computed tomography (CT), into the diagnostic pathway, although these also have their limitations. A correct, detailed interpretation of the ultrasound scan results requires an experienced diagnostician, whereas the prevalent use of CT would increase the cost and contribute to abundant radiation exposure [1, 11].
To find a specific marker for AA, recent studies considered an association between an increase in serum total bilirubin concentration (TBil) and the development of complex (gangrenous or perforated) AA [7, 10, 13, 14]. Other studies have attempted to evaluate the usefulness of hyperbilirubinemia in distinguishing AA from other conditions presenting with similar clinical symptoms, and the outcomes confirmed the hypothesis [2, 15]. Moreover, in the face of growing evidence for non-surgical treatment of uncomplicated AA the search for a clinically useful marker to establish the severity of AA seems obligatory [16].
Aim
The aim of the present study was to examine the association between increased serum total bilirubin concentration and the development of complex AA (concurrent with perforation and/or necrosis) [13].
Material and methods
This retrospective study involved all patients admitted to the Surgical Department between January 2015 and December 2017 with clinical presentations of AA. Collected data covered patients’ laboratory values, results of imaging studies, body mass index (BMI; height and weight), and reports regarding complications and treatment during the hospital stay. A total of 169 patients with a presumptive diagnosis of AA who underwent an operation were included in this study. Patients were scheduled to undergo emergency laparotomy/laparoscopy either by elevated inflammatory markers and/or imaging studies with suspicion of acute appendicitis or by peritonitis signs during physical examination. Prior to admission, female patients were consulted by a gynecologist and those in reproductive age also had blood tests for human chorionic gonadotropin level.
The patients were then classified into 3 consecutive groups according to appendix histopathology and operative reports. The first group comprised patients with simple inflammation understood as simple and suppurative AA. The second group included patients with complex and thus gangrenous or perforated AA. Patients with a final diagnosis other than AA constituted the third group.
Statistical analysis
The Kruskal-Wallis and Mann-Whitney U tests, Fisher’s exact test, Spearman correlation coefficient and logistic regression were used. A p-value < 0.05 was considered to be statistically significant. Confidence intervals (CIs) were set at 95%. A receiver operating characteristics (ROC) curve was plotted for perforated and simple appendicitis in terms of serum bilirubin concentration. The laboratory norm for total bilirubin concentration in our hospital is set at < 1.2 mg/dl. Analyses were computed using Statistica version 13.1 software (Dell Inc., Tulsa, USA). The study protocol was approved by the local ethics committee and has therefore been performed in accordance with the Helsinki Declaration of 1975.
Results
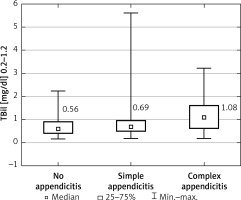
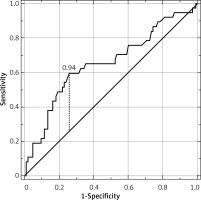
Patients’ characteristics are shown in Table I. Appendicitis was confirmed histologically in 124 of 169 patients (73.4%). Eighty-three patients were diagnosed with simple AA (49.1% of all), and the remaining 41 (24.3%) patients developed complex inflammation. The median serum total bilirubin concentration for all 3 groups was 0.56, 0.69, and 1.08 mg/dl for non-appendicitis, simple AA, and complex AA, respectively (p = 0.020) (Figure 1). The C-reactive protein (CRP) concentration was positively correlated with TBil (R = 0.271, p < 0.001) in all patients. The optimal cut-off point for TBil for prediction of complex appendicitis was 0.94 mg/dl (Figure 2) with a sensitivity of 59.5%, specificity of 74.3%, positive predictive value (PPV) of 44.9%, and negative predictive value (NPV) of 83.9%. The area under the curve (AUC) was 0.652 (95% CI: 0.543–0.761), p = 0.006. The ROC curve analysis for estimation of simple AA was not statistically significant (p = 0.067). Analyses of the percentage of patients with complex appendicitis with hyperbilirubinemia compared to the rest of the patients with increased TBil (with simple appendicitis or without it) were statistically significant (p = 0.003) with sensitivity of 43.2% and specificity of 82.9%. Analyses of the percentage of patients with hyperbilirubinemia with or without appendicitis (either form) were not statistically significant (p = 0.830). The increase in the patients’ TBil, white blood cells (WBC), and CRP concentrations is displayed in Table II. Multivariable analysis revealed that TBil above 0.94 mg/dl was an independent predictor for complex AA among WBC and CRP (Table III). The overall postoperative complication rate for all patients was 13.6% (9.3% in simple AA, 29.3% in complex and 6.7% in non-AA group). Postoperative complications were not significantly correlated with bilirubin concentration (p = 0.525).
Table I
Baseline characteristics of the study group
Table II
Quantity of patients with increased biochemical markers and patients with increased bilirubin above optimal cut-off point established by ROC analysis
Table III
Multivariable analysis of factors independently associated with complex AA
| Parameter | Multivariable | |
|---|---|---|
| OR (95% CI) | P-value | |
| CRP* [mg/l] | 1.10 (1.03–1.17) | 0.001 |
| TBil ≥ 0.94 mg/dl | 1.67 (1.01–2.59) | 0.020 |
| WBC [103/μl] | 1.11 (1.01–1.23) | 0.040 |
Other conditions that affected patients primarily diagnosed with AA were gynecological conditions (n = 11; displayed in Table IV), lymphadenitis (n = 6 of which 4 were mesenteric lymphadenitis), inflammation of the surrounding soft tissues (n = 6), mucinous cystadenoma of the appendix (n = 3), inflammation associated with a G1 NET tumor (n = 2), and chronic inflammation of the appendix (n = 2). Apart from that a single case of cholecystitis, infiltrating cecal tumor, Meckel’s diverticulitis, stomach perforation, colon diverticulitis infiltrating the appendix, adhesive bowel disease, appendiceal cyst, infiltration of the lymphoma, and abdominal pain 10 months after caesarean section were noted. The cause of symptoms was not found in 6 patients. Seventy-one patients developed periappendicitis.
Discussion
Impairment of hepatic function as a result of septic conditions has been well described [2]. Jaundice was observed in patients with AA over 60 years ago [5]. The putative mechanism of hyperbilirubinemia in AA is associated with bacteria and bacterial toxins carried by the portal vein to the liver. Both the cytotoxicity of the bacterial product and the inflammatory response lead to dysfunction of the hepatocytes, which affects bilirubin secretion [2, 15, 17]. Other studies [7] have indicated that hemolysis, caused by a septic condition, is an important mechanism causing hyperbilirubinemia. This statement explains the lack of increase in liver transaminases in 95–98% of the patients [2, 18].
Recently, an increasing amount of studies have examined the association between hyperbilirubinemia and inflammation of the vermiform appendix, some of which found bilirubin to be a specific marker for appendiceal perforation. According to D’Souza et al. [2], Muller et al. [7], Giordano et al. [10] and Silva et al. [19] the specificity, sensitivity, PPV, and NPV of hyperbilirubinemia compared for simple versus perforated appendicitis were 70–82% [2, 7, 10, 19], 48–70% [2, 7, 10, 19], 18–47% [2, 7], and 92–93% [2, 7], respectively. For comparison, results pooled in this research were 74.3%, 59.5%, 44.9%, and 83.9%, respectively. Whereas the specificity, sensitivity and PPV nearly matched those found in the literature [2, 7, 10, 19] the NPV was slightly lower.
With regards to final alternative diagnoses, the most common were neoplasms and gynecological conditions as well as other inflammatory aliments such as adnexitis or lymphadenitis. A common co-existing morbidity with AA pathology was periappendicitis, defined as inflammation of the surrounding soft tissue (neutrophilic infiltration). A probable explanation for the suspicion of appendicitis is the misleading signs of peritonitis caused mostly by free blood in the abdomen. Even a small amount of blood can cause irritation of the peritoneum. Second, easy access and abuse of antibiotics may alleviate the symptoms of appendicitis and lower inflammation markers prior to hospital admission in an emergency. In such cases, the distinction of acute appendicitis by surgeons becomes more and more difficult in our time.
Evidence suggests that laparoscopic appendectomy appears to be a method of choice in most patients presenting with the symptoms of AA [6, 16]. However, recent studies such as the APPAC randomized clinical trial published by Salminen et al. [20] and a meta-analysis by Podda et al. [16], which included more than 3500 patients with suspicion of AA, have shown that antibiotic therapy for image-proven uncomplicated appendicitis is a viable treatment option compared to surgery. Podda et al. [16] reported antibiotic treatment to have a failure rate of 27.7% at 1-year follow-up along with lower complication-free treatment success (no postoperative complications, adverse events, or treatment failure occurring – 67.2% for conservative vs. 82.3% for surgical management). Additionally, a decreased rate of postintervention complications (7.1% vs. 14.5%) and reduced cost were noted. They also found that the differences between patients with uncomplicated AA treated primarily surgically and conservatively, who required a second hospitalization due to recurrent appendicitis, were not statistically significant, as far as increased perforation rate and the higher risk of postoperative complications were concerned. Currently the only appreciable examination to differentiate simple and complex AA is CT [20, 21], but its use in every patient cannot be justified as it requires exposure to a considerable dose of radiation along with being high in cost for the hospital [3].
The study has several limitations. Firstly, it is a retrospective study with a moderate number of patients. Secondly, due to the retrospective nature of the study, the total bilirubin concentration could not be examined for the division of its components (unconjugated and conjugated). Lastly, there is also a concern regarding potential inclusion of patients with Gilbert’s syndrome in the study group. On the one hand, it could influence outcomes. On the other hand, inclusion of those patients corresponds to a more natural situation. Not all patients are aware of having Gilbert’s syndrome and there are no methods to confirm this on the day of the emergency admittance to the hospital.
Conclusions
As far as the utility for serum bilirubin concentration in the diagnoses of complex AA is concerned, the results were equivocal. On the one hand, the poor sensitivity and PPV indicated that as a standalone marker, serum bilirubin concentration is not enough to predict a perforation; on the other hand, the obtained high negative predictive value can help in the selection of patients who do not require immediate surgery. Some of them can be treated conservatively and/or subjected to further observation. Owing to a satisfactory specificity, a presumptive diagnosis of complex AA can be confirmed. Undoubtedly, an increase in TBil is not an anomalous biochemical test outcome in AA and should be considered by physicians as one of the markers in a more severe condition. Bilirubin can be used as an additional indicator enabling the selection of a group of patients who will not require urgent surgery. This marker is relatively cheap and available in every emergency department, and its value turns out to be clinically useful.