Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
OBSTETRICS AND GYNAECOLOGY / SYSTEMATIC REVIEW/META-ANALYSIS
Serum vitamin D deficiency and risk of gestational diabetes mellitus: a meta-analysis
1
Maternity Department, W.F. Maternity and Child Care Hospital, Weicheng District, China
2
Neonatology Department, Weifang Medical University, Weicheng District, China
3
Pharmacy Department, W.F. Maternity and Child Care Hospital, Weifang, Weicheng District, China
4
Public Computer Center, Weifang Medical University, Weifang, Kuiwen District, China
Submission date: 2019-09-20
Final revision date: 2020-02-13
Acceptance date: 2020-02-23
Online publication date: 2020-04-15
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):742-751
KEYWORDS
TOPICS
ABSTRACT
Introduction:
This meta-analysis was performed to confirm the relationship of gestational diabetes mellitus (GDM) and vitamin D.
Material and methods:
PubMed and CNKI databases were searched for relevant articles. Standard mean difference (SMD) along with 95% CI was used to compare vitamin D level between women with GDM and healthy subjects. The correlation coefficient between the vitamin D and homeostasis model assessment-insulin resistance index (HOMA-IR) was analyzed.
Results:
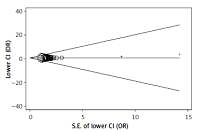
The vitamin D level of GDM subjects was much lower than healthy subjects (SMD = –0.71, 95% CI: –0.91, –0.50). Vitamin D deficiency was associated with high risk of GDM (OR = 1.15, 95% CI: 1.07–1.23). Vitamin D was negatively correlated with HOMA-IR (r = –0.62, 95% CI: –0.85, –0.39). The analysis showed no publication bias (Egger’s: p = 0.197; Begg’s: p = 0.786).
Conclusions:
Vitamin D is closely associated with the onset of GDM.
This meta-analysis was performed to confirm the relationship of gestational diabetes mellitus (GDM) and vitamin D.
Material and methods:
PubMed and CNKI databases were searched for relevant articles. Standard mean difference (SMD) along with 95% CI was used to compare vitamin D level between women with GDM and healthy subjects. The correlation coefficient between the vitamin D and homeostasis model assessment-insulin resistance index (HOMA-IR) was analyzed.
Results:
The vitamin D level of GDM subjects was much lower than healthy subjects (SMD = –0.71, 95% CI: –0.91, –0.50). Vitamin D deficiency was associated with high risk of GDM (OR = 1.15, 95% CI: 1.07–1.23). Vitamin D was negatively correlated with HOMA-IR (r = –0.62, 95% CI: –0.85, –0.39). The analysis showed no publication bias (Egger’s: p = 0.197; Begg’s: p = 0.786).
Conclusions:
Vitamin D is closely associated with the onset of GDM.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.