Introduction
Catheter ablation has become a standard therapeutic option in patients with symptomatic, drug-refractory atrial fibrillation (AF) [1–3]. Pulmonary vein isolation (PVI), confirmed with documented bidirectional conduction block between the left atrium (LA) and pulmonary veins (PVs), is the cornerstone of AF ablation [4]. The conventional approach, i.e. circumferential isolation of ipsilateral PVs achieved point-by-point with an irrigated single-tip radiofrequency (RF) catheter (usually with the support of a three-dimensional electroanatomical system), is a time-consuming procedure. To facilitate the AF ablation procedure, several “single-shot” devices have been developed [5–8], leading to substantial shortening of the procedure time with a safety profile and long-term success rate comparable to classical point-by-point PVI [9–14]. Silent cerebral infarcts (SCIs), detected by diffusion-weighted magnetic resonance imaging (DW-MRI), associated with many types of cardiovascular interventions, have been widely described [15–18], but they are usually linked with PVI procedures [19–24] due to the specific target areas in the left atrium and higher initial risk of thromboembolic events in the AF population. In some of the single-shot techniques, the reported increased incidence of SCIs, detected with post-procedural DW-MRI, is of particular concern [21, 22].
In our study, we aimed to compare the incidence of SCIs detected with DW-MRI following AF ablation with four different technologies: classical point-by-point ablation with single-tip irrigated RF catheter and three different single-shot devices – non-irrigated multipolar phased-RF catheter, second-generation cryoballoon and multipolar irrigated RF catheter.
Material and methods
Study population
One hundred and four patients of mean age 59.9 ±9.5 years (68 males) with symptomatic AF, referred for ablation in our center, were included in the study. Exclusion criteria were previous AF ablation, enlarged (> 50 mm) left atrium (LA), left atrial thrombus, long-lasting persistent AF, substantial valvular heart disease, left ventricular (LV) ejection fraction ≤ 40%, heart failure (NYHA class III or IV), history of myocardial infarction or episode of unstable angina within the past 6 months, thyroid dysfunction, contraindication to oral anticoagulants, pregnancy and contraindication to magnetic resonance imaging. The distribution of factors potentially related to thromboembolic risk (comorbidities, LA diameter, persistent form of AF) was well balanced between the groups (Table I). Arterial hypertension was the most frequent comorbidity in all groups, which is consistent with other observations regarding the AF population [23–27]. The ablation procedure was performed with irrigated radiofrequency point-by-point technique (RF group) in 24 patients, and with 3 different single-shot techniques: a non-irrigated duty-cycled phased-RF catheter (PVAC) in 46 patients, a second-generation cryoballoon (CB) in 24 patients and with an irrigated multipolar RF catheter (nMARQ) in 10 patients. The choice of the ablation technique was at the operator’s discretion. Most of the procedures (RF: 17/24 (71%), PVAC: 41/46 (89%), CB: 21/24 (88%), nMARQ: 8/10 (80%)) were performed by A.G., with no significant differences between the groups (p = 0.24). In all patients DW-MRI was performed before and within 24 h after the ablation procedure to evaluate the incidence of SCIs. Procedural parameters, complication rate and post-procedural SCI incidence were compared between the groups. Patient characteristics are presented in Table I. The study protocol was approved by the institutional review board and written informed consent was signed by all patients.
Table I
Characteristics of the patients
| Parameter | RF group (n = 24) | PVAC group (n = 46) | CB group (n = 24) | nMARQ group (n = 10) | P-value |
|---|---|---|---|---|---|
| Baseline characteristics: | |||||
| Age [years]* | 58.0 ±9.15 | 60.7 ±8.8 | 59.7 ±10.3 | 61.4 ±12.1 | 0.49 |
| Male | 17 (71%) | 34 (74%) | 19 (79%) | 8 (80%) | 0.89 |
| BMI [kg/m2]* | 28.6 ±2.4 | 27.5 ±2.6 | 26.8 ±6.0 | 27.6 ±2.3 | 0.42 |
| Heart failure | 1 (4.2%) | 1 (2.2%) | 0 (0%) | 1 (10%) | 0.43 |
| Hypertension | 18 (75%) | 32 (70%) | 18 (75%) | 8 (80%) | 0.89 |
| Diabetes | 4 (17%) | 10 (22%) | 6 (25%) | 2 (20%) | 0.91 |
| Previous stroke | 2 (8.3%) | 2 (4.3%) | 2 (8.3%) | 0 (0%) | 0.60 |
| CHA2DS2-VASc score* | 1.6 ±1.6 | 1.7 ±1.2 | 1.8 ±1.4 | 1.9 ±0.9 | 0.74 |
| LA diameter [mm]* | 43.0 ±4.6 | 42.8 ±3.3 | 42.5 ±2.6 | 43.7 ±2.8 | 0.48 |
| LV ejection fraction (%)* | 62.9 ±5.0 | 60.3 ±4.7 | 63.2 ±5.2 | 60.2 ±5.2 | 0.07 |
| Persistent AF | 3 (13%) | 3 (7%) | 2 (8%) | 1 (10%) | 0.87 |
| Procedural characteristics: | |||||
| Procedure time [min]* | 167.1 ±30.7 | 110.5 ±19.7 | 106.0 ±20.2 | 141.5 ±24.3 | < 0.001 |
| LA dwell time [min]* | 101.5 ±22.5 | 53.9 ±8.0 | 56.0 ±9.0 | 97.0 ±20.9 | < 0.001 |
| Fluoro time [min]* | 22.7 ±10.6 | 15.9 ±5.9 | 16.3 ±8.5 | 15.3 ±5.2 | 0.048 |
| Complications (minor) | 3 (13%) | 3 (7%) | 2 (8%) | 1 (10%) | 0.87 |
Periprocedural management
A computed tomography (CT) scan or contrast venography was performed to evaluate pulmonary veins and LA anatomy. All patients were on oral anticoagulation prior to the procedure. For vitamin K antagonists (VKAs), an uninterrupted anticoagulation strategy was applied with therapeutic values of international normalized ratio (INR) [28]. For novel oral anticoagulants (NOACs), an interrupted regimen was accepted, with the preceding dose 12 to 24 h before, and the following dose 6 h after the ablation procedure. In all patients, transesophageal echocardiography (TEE) was performed before the procedure to exclude left atrial thrombus.
Ablation procedure
All procedures were performed under conscious sedation with local anesthesia. After two punctures in the right femoral vein (three punctures in the group of patients undergoing point-by-point ablation) a steerable quadripolar diagnostic catheter was inserted into the coronary sinus (CS). Transseptal puncture was performed under fluoroscopic guidance using the 10-French (SL0, St. Jude Medical) sheath, which was continuously flushed with heparinized saline (1 U/ml) throughout the procedure. A loading dose (5000 IU) of heparin was given to all patients before the transseptal puncture. Immediately after crossing the septum, an additional weight-dependent dose of heparin was given to reach 70 IU/kg. The total dose was titrated based on activated clotting time (ACT) value (checked at 20-minute intervals) to maintain the ACT level above 300 s throughout the procedure.
In the RF group, the AF ablation procedure was performed using the 3D electroanatomical system CARTO (Biosense Webster Inc, Diamond Bar, CA, USA) and a contact-force ablation catheter, SmartTouch (Biosense Webster Inc, Diamond Bar, CA, USA), in 15 patients; in 9 patients, a standard irrigated catheter was used (ThermoCool, Biosense Webster Inc, Diamond Bar, CA, USA). In all patients, a circular diagnostic catheter – Lasso (Biosense Webster Inc, Diamond Bar, CA, USA) – was used for 3D mapping of the left atrium. The Lasso catheter was introduced via the separate transseptal sheath in all patients to eliminate the need for catheter replacement during the procedure. The RF generator – EP Shuttle (Stockert GmbH, Freiburg, Germany) – was set at power control mode with maximum power of 35 W (limited to 25 W at posterior wall). In all patients, wide antral circumferential ablation was performed with both ipsilateral veins isolated together. In 12 (50%) patients, an additional substrate modification was performed: mitral isthmus and roof lines in 9 patients and in the cavo-tricuspid isthmus (CTI) line in 4 patients with a history of concomitant typical atrial flutter (1 patient had both LA and CTI lines performed together with PVI).
In the PVAC group, a circular multipolar catheter (PVAC, Medtronic, Minneapolis, USA) and dedicated multichannel duty-cycled phased-RF energy generator (GENius, Medtronic, Minneapolis, USA) were used, as described elsewhere [6, 7]. A modified PVAC Gold catheter was used in the last twelve cases. Phased-RF energy applications of 60 s were delivered with a unipolar/bipolar ratio of either 4 : 1 (8 W) or 2 : 1 (10 W), until pulmonary vein isolation was achieved.
In the CB group, all procedures were performed with a 28 mm second-generation cryoballoon (Arctic Front Advance, Medtronic, Minneapolis, MN, USA), as described elsewhere [5, 11]. All pulmonary veins were mapped with an inner lumen mapping catheter – Achieve 20 mm (Medtronic, Minneapolis, MN, USA). Vessel occlusion was considered optimal when selective contrast injection showed no backflow to the atrium. Once occlusion was documented; cryoapplication was commenced. In the first 8 patients double 4-minute freeze was applied. In all others, a single 3-minute freeze strategy was applied.
In the nMARQ group the ablation process was similar to the PVAC technique. The main difference was the use of a 3D electroanatomical system (CARTO, Biosense Webster Inc, Diamond Bar, CA, USA) in order to create a three-dimensional anatomical map of the left atrium and to confirm the positioning of the irrigated circular nMARQ catheter against the PV ostia. The power of unipolar RF application was set at 20 W (15 W at posterior wall) and maximum time of a single application was 30 s.
In all procedures performed with single-shot devices (PVAC, CB and nMARQ) pulmonary veins were the only target, with no additional substrate modification. In all patients, at the end of the procedure bidirectional PV isolation was confirmed with either a Lasso, PVAC, Achieve or nMARQ catheter. The choice of the AF ablation technique was at the operator’s discretion. In all cases, the procedure time, fluoroscopy time and the duration of the catheters’ dwell in the left atrium (LA dwell time) were recorded. Transthoracic echocardiogram was performed after the procedure to exclude pericardial effusion in all patients.
Diffusion-weighted MRI
Magnetic resonance imaging (1.5 Tesla Siemens Avanto) was performed the day before and 24–48 h after the ablation procedure in all patients, as described before [18]. Acute ischemic lesions were defined as focal diffusion anomalies – “bright signals”. Additionally, the apparent diffusion coefficient (ADC) was measured to support image analysis in equivocal cases. All MRI scans were analyzed by a certified radiologist.
Statistical analysis
Statistical analysis was carried out using appropriate statistical tests and Statistica 13.3, TIBCO Software Inc. (StatSoft, USA). The values of the parameters were presented as arithmetic means and their standard deviations (SD), medians, minimum and maximum value, and the range of variability. Normal distribution of continuous variables was tested using the Shapiro-Wilk test. The Student t-test or Mann-Whitney U-test or Kruskal-Wallis ANOVA analysis for independent variables was used for intergroup comparisons. The distribution of discrete variables in groups was compared with Pearson’s χ2 test or Fisher’s exact test. Logistic regression models were fitted to identify risk factors associated with the occurrence of SCI. From these models, adjusted odds ratios (OR) and 95% confidence intervals were derived; corresponding p-values were those from Wald’s test. Goodness of fit was checked using Hosmer and Lemeshow’s test. The error was set at 5% and significance at p-value < 0.05.
Results
Procedural parameters and complications
Procedure (167.1 vs. 110.5 vs. 106.0 vs. 141.5 min, p < 0.001), LA dwell (101.5 vs. 53.9 vs. 56.0 vs. 97.0 min, p < 0.001) and fluoroscopy (22.7 vs. 15.9 vs. 16.3 vs. 15.3 min, p = 0.048) times differed significantly within the RF, PVAC, CB and nMARQ groups, respectively. When compared to classical RF point-by-point PVI, all three single-shot techniques had significantly shorter procedure time: RF vs. PVAC (p < 0.001), RF vs. CB (p < 0.001) and RF vs. nMARQ (p = 0.027). The LA dwell time was significantly shorter in PVAC (p < 0.001) and CB (p < 0.001) groups, but not in the nMARQ group (p = 0.59). Similarly, the fluoroscopy time was significantly reduced with PVAC (p < 0.001) and CB (p = 0.026), and slightly shorter in the nMARQ group (p = 0.076).
There were no major complications of the ablation procedure. Minor complications occurred in 9 (8.6%) patients and involved local groin hematoma in 8 patients and one pseudoaneurysm of the right femoral artery, which was effectively treated with ultrasound-guided compression. There was no significant difference in complication rate between the groups (p = 0.87). The summary of procedural parameters and complications is presented in Table I.
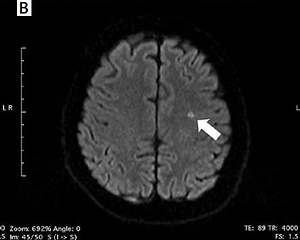
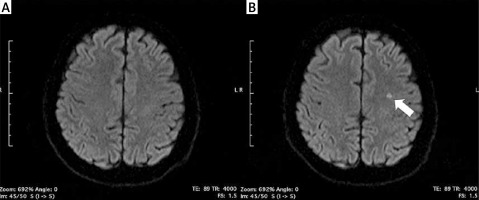
Incidence of silent cerebral infarcts following the ablation procedure
New-onset SCIs detected by post-procedural DW-MRI occurred in 4 (16.7%) patients in the RF group, 7 (15.2%) patients in the PVAC group, and in 1 patient both in CB (4.2%) and nMARQ (10%) groups (Figure 1). All of the revealed infarcts were clinically silent and there were no neurological symptoms following the procedure. In a univariable analysis the need for intraprocedural cardioversion (p = 0.002), persistent form of arrhythmia (p = 0.007), procedure time (p = 0.02), and lower LV ejection fraction (p = 0.03) were identified as risk factors of new-onset SCIs (Table II).
Table II
Univariable analysis of SCI incidence risk factors
| Parameter | SCI (–) (n = 91) | SCI (+) (n = 13) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Age [years]* | 60.2 ±9.8 | 57.8 ±6.8 | 0.97 (0.9–1.03) | 0.38 |
| Male | 66 (73%) | 12 (92%) | 4.5 (0.6–36.8) | 0.16 |
| BMI [kg/m2]* | 27.6 ±3.7 | 27.3 ±2.6 | 0.98 (0.8–1.1) | 0.77 |
| CHA2DS2-VASc score* | 1.77 ±1.4 | 1.46 ±0.97 | 0.82 (0.5–1.3) | 0.43 |
| LA diameter [mm]* | 42.8 ±3.5 | 43.4 ±3.1 | 1.05 (0.9–1.3) | 0.57 |
| LV ejection fraction (%)* | 62.0 ±5.0 | 58.5 ±4.9 | 0.88 (0.8–1.0) | 0.03 |
| Left common PV trunk | 15 (17%) | 3 (23%) | 1.5 (0.4–6.1) | 0.57 |
| Persistent AF | 5 (6%) | 4 (31%) | 2.8 (1.3–5.8) | 0.007 |
| CRP [mg/l]* | 2.2 ±4.5 | 2.7 ±5.7 | 1.0 (0.9–1.1) | 0.71 |
| BNP [pg/ml]* | 73.7 ±67.0 | 55.9 ±53.0 | 0.99 (0.98–1.0) | 0.36 |
| Urea [mg/dl]* | 39.1 ±9.0 | 43.2 ±8.7 | 1.05 (1.0–1.1) | 0.13 |
| Creatinine [mg/dl]* | 0.95 ±0.2 | 0.98 ±0.23 | 2.2 (0.2–32.3) | 0.56 |
| eGFR [ml/min]* | 82.6 ±15.7 | 81.3 ±15.23 | 0.99 (0.9–1.03) | 0.77 |
| Procedure time [min]* | 122.3 ±31.9 | 147.7 ±39.3 | 1.02 (1.0–1.04) | 0.02 |
| LA dwell time [min]* | 68.3 ±24.5 | 77.8 ±32.8 | 1.0 (0.99–1.03) | 0.22 |
| DC cardioversion | 9 (10%) | 6 (46%) | 7.7 (2.1–28.0) | 0.002 |
| ACT during procedure* | 354.0 ±51.0 | 332.9 ±43.7 | 0.99 (0.98–1.0) | 0.16 |
SCI – silent cerebral infarcts, OR – odds ratio, CI – confidence interval, BMI – body mass index, LA – left atrium, LV – left ventricle, PV – pulmonary vein, AF – atrial fibrillation, CRP – C-reactive protein, BNP – brain natriuretic peptide, eGFR – estimated glomerular filtration rate, DC – direct current, ACT – activated clotting time.
Discussion
Procedural parameters and complications
Single-shot devices dedicated for AF ablation were developed to simplify PVI and reduce the procedure time. We demonstrated significantly shorter procedural duration, LA dwell and fluoroscopy times with two single-shot techniques – phased RF multipolar catheter (PVAC) and second-generation cryoballoon – compared to classical point-by-point RF. For the irrigated multipolar RF catheter (nMARQ) only reduction of the procedure duration was demonstrated, without a significant change in fluoroscopy and LA dwell time. There are two possible explanations for this difference. First, our results regarding a significant reduction of procedure time with PVAC and CB are in line with previous reports [9–13]. Second, the nMARQ group included the first 10 patients ablated with this technique in our center, and thus the operator’s caution with the new technology can explain the relatively longer procedure duration. Interestingly, in a recently published report comparing PVAC and nMARQ catheters, both the procedure and fluoroscopy times were significantly shorter with the latter technique [12].
The observed rate of minor complications did not differ between the groups and was comparable with previously reported rates [5–14]. This is an important remark, since the main reason for development of single-shot technologies was to simplify the procedure without compromising its safety.
Incidence of silent cerebral infarcts following the ablation procedure
In our study, we compared the incidence of silent cerebral infarcts detected by DW-MRI following AF ablation performed with four different technologies.
This phenomenon, associated with cardiovascular interventions, was described predominantly following catheter ablation procedures [18–24]. The clinical significance of SCIs, by definition asymptomatic, is being discussed, and there are data linking this phenomenon with dementia and gradual cognitive decline [29, 30]. Furthermore, SCIs can be considered as a marker of thrombogenicity of cardiovascular interventions. Fortunately, clinically apparent stroke does not happen frequently enough during most cardiovascular procedures to help us to analyze its risk factors, except in very large cohorts. In our opinion, expressed before [18], SCIs can serve as a “substitute” of stroke, permitting the search for potential patient- and procedure-dependent risk factors in studies involving smaller numbers of patients.
In our group, there was non-significantly lower incidence of SCIs in the group of patients ablated using second-generation cryoballoon, which is in line with the previously published data [21, 22]. Interestingly, the rate of SCIs following irrigated RF point-by-point isolation was higher than previously reported [19–22], and comparable with phased-RF non-irrigated technique. The possible explanation of this finding is that in 9 patients in the point-by-point group, additional left atrial lines were performed, which might have an influence on the SCI incidence directly, together with the prolonged catheter dwell in the left atrium. In contrast, in the three groups ablated using single-shot techniques there were no additional ablation lines beyond PVI. In fact, in 3 out of 4 patients with new-onset post-procedural SCIs in the RF point-by-point group, additional left atrial lines were performed. Taking into account patients with PVI alone, the incidence of SCIs in the RF group would be much lower and similar to that observed in the CB group.
Several identified risk factors of SCI following AF ablation have been reported: age [31], persistent form of arrhythmia [32] spontaneous echocardiographic contrast in TEE examination [33, 34], enlarged left atrium [35], and cardioversion during the ablation procedure [20].
In our study, univariable analysis revealed the procedure duration, lower LV ejection fraction, persistent form of arrhythmia and the need for an intraprocedural DC cardioversion as the independent predictors of SCI occurrence (Table II). The prolonged procedure time might itself have an influence on the risk of silent cerebral infarcts. The relations between SCIs and the type of arrhythmia [32] and electrical cardioversion [20] during the procedure (potentially unleashing left atrial thrombi formed in the left atrium) have been described. The relation between lower LV ejection fraction and the new-onset SCIs can be explained by a preexisting prothrombotic state in patients with heart failure [36]; however, to the best of our knowledge, there are no previous reports on lower LV ejection fraction as a risk factor of periprocedural silent cerebral infarcts.
Last but not least, we still do not know whether this phenomenon is clinically relevant, since in the majority of cases the post-procedural SCIs were no longer detectable in DW-MRI performed several months after the index procedure [37].
There are several limitations of our study. First, it is a single-center, non-randomized study, performed on a relatively small number of patients, and the operators had much less experience with one of the techniques (nMARQ), which may constrain our capacity to draw substantial conclusions. Second, in 9 patients in the RF group additional left atrial lines were performed, which might have influenced SCI incidence and procedure duration. Third, in the last twelve cases in the PVAC group a modified version of the catheter was used, which might have had an impact on SCI incidence, though probably not on the procedural parameters.
In conclusion, we have compared the incidence of silent cerebral infarcts following atrial fibrillation ablation with four different technologies and analyzed the possible risk factors. A prolonged procedure time, lower LV ejection fraction, persistent form of arrhythmia and a need for intraprocedural cardioversion increase the risk of silent cerebral infarcts following the ablation procedure.