BACKGROUND
TOBACCO CONSUMPTION IN GENERAL AND DURING PREGNANCY
In Switzerland, approximatively 27% of the population smokes regularly (almost 2 million), causing 9500 premature death every year (Office fédéral de la santé publique (OFSP), 2019). Today, active and passive smoking’s risks are well established from the scientific community. Notably, tobacco consumption causes different types of cancers, cardiovascular diseases, pulmonary diseases, and other conditions such as diabetes (Cornuz et al., 2015). Tobacco also harms pregnant women and their fetus. During pregnancy, maternal smoking increases the risk of spontaneous miscarriage, ectopic pregnancy, preterm birth, small for gestational age, fetal abnormalities, and stillbirth (not exhaustive) (Benachi et al., 2014; Cornuz et al., 2015; Diamanti et al., 2019; Godding, 2010; Meernik & Goldstein, 2015; Rumrich et al., 2020). During the postnatal period, smoking during pregnancy can also increase the risk of neonatal death and SIDS (sudden infant death syndrome) (Cornuz et al., 2015; Diamanti et al., 2019; Meernik & Goldstein, 2015). It has been shown that carbon monoxide (CO) and nicotine, two main components of smoked tobacco, can have negative effects on the fetus (Diamanti et al., 2019; Godding, 2010). CO, when inhaled, has a high blood affinity resulting in hypoxia, fetal higher blood pressure and growth restriction for the fetus (Diamanti et al., 2019; Godding, 2010), resulting in a reduction of the fetus’ movement in utero and death. Nicotine has a neuronal impact, leading to abnormal neurotransmitter function (Diamanti et al., 2019).
Smoking cessation during pregnancy therefore appears to be a public health priority. Spontaneously or with aid, many women quit smoking during pregnancy. In Switzerland, 56.6% of women smokers report quitting smoking when knowing they are pregnant (OFSP, 2018). Nevertheless, the prevalence of tobacco consumption during pregnancy ranges from below 10% up to 48%, depending on the pregnancy period for which statistics were done (Diamanti et al., 2019). In Switzerland, the prevalence of smoking in pregnancy varies between 7% (OFSP, 2018), 14% (Keller et al., 2009), and up to 21.7% (Dupraz et al., 2013). Moreover, tobacco relapse after birth, defined as a resumption of tobacco consumption during the first year following birth, is frequent, leaving unchanged the risk of smoking for the mother and her entourage. Notley et al. (2015) reported that from 70% up to 85% of women who stopped smoking during pregnancy will resume their consumption, and almost 75% during the first 6 months after birth. Studies by Willi et al. (2006) and by Meernik and Goldstein (2015) found that 90% of women would resume after 12 months. A recent study by Diamanti et al. (2019) found a prevalence of tobacco relapse between 47% and 63% six months after birth. Thus, determining what makes it easier for pregnant women to definitively stop smoking is an important issue.
DETERMINANTS OF SMOKING CESSATION AND POST-PARTUM RELAPSE
Various types of determinants are related to smoking cessation during pregnancy: physiological aspects, socio-demographics, and psychological factors. It has been shown that spontaneous quitting is facilitated by the fact that women’s metabolism is modified during pregnancy. For example, Espiand-Marçais et al. (2014) mentioned an augmentation of nicotine metabolism of 140% at the end of the pregnancy. Riaz et al. (2018) conducted a meta-analysis to find out socio-economic determinants and contextual factors associated with smoking cessation during pregnancy. In 54 studies reviewed, including 505 584 women, they were able to identify that predictors of cessation are: older maternal age, higher socio-economic status, overseas maternal birth, living with partner or married, lower exposure to passive smoking, low cigarette dependence, low exposure to second-hand smoking, not drinking alcohol before and/or during pregnancy, primiparity, perceived adequate prenatal care, planned breastfeeding, good mental health during pregnancy and higher levels of self-efficacy for quitting. This is consistent with another report based on a cohort study (El-Khoury et al., 2017).
Aside from socio-demographics variables, motivational variables are interesting to consider because they can be targeted by behaviour change interventions. Contrary to the socio-economical level of the person, which is difficult to modify, there are techniques to boost motivation or to increase the perceived ability to do the behaviour if the person believes it is too difficult (see for example Brown et al., 2019, for a review of behaviour change techniques for preventing postpartum tobacco relapse).
In order to know which behaviour change techniques are appropriate, it is essential to assess the barriers of the behaviour first (Crutzen & Peters, 2019; Green & Kreuter, 2005). The theory of planned behavior (TPB; Ajzen, 1985, 1991) is a prominent theory in psychology for explaining health behaviours, and interventions based on this theory have been shown to be effective (Steinmetz et al., 2016). The TPB has been applied widely to the topic of smoking in general (see Topa & Moriano, 2010, for a meta-analysis), but more rarely to issues surrounding pregnancy and tobacco consumption. In a theoretical paper, Gantt (2001) reviewed the TPB and argued that it is highly relevant and applicable to the issue of postpartum smoking relapse. Ben Natan et al. (2010) studied the links between attitude, subjective norms and perceived behavioural control and intention to smoke during pregnancy, and found that the three predictors do explain variance intention as predicted by the TPB. The cross-sectional design and the use of intention as the DV are limitations of this study, calling for confirmatory data using behavioural data to circumvent the intention-behaviour gap (Sheeran, 2002). Moreover, their sample is not solely composed of pregnant women. Thus for some of the respondents, the intention question was highly hypothetical (e.g. of intention item: “I will make every effort to quit smoking the moment I discover that I am pregnant”). De Wilde et al. (2017) measured TPB beliefs among pregnant women and detected correlations between attitude, subjective norms and perceived behavioural control and tobacco consumption during the pregnancy. In their data, intention to quit smoking was not predicted by TPB beliefs after controlling for age and education. In this paper the authors did not explore the link with postpartum tobacco consumption. To the best of our knowledge, the only study using the TPB framework to understand postpartum tobacco consumption is that of Godin et al. (1992). Their results highlight the fact that perceived behavioural control seems to be the most important predictor of behaviour in this situation (4 months after childbirth). This rare reference is dated from nearly 30 years ago, and with the evolution of the perception of smoking in society, it seems important to reassess whether the results are still valid nowadays. Alamar and Glantz (2006) found that the social unacceptability index increased by 10.3% in only three years (1996-1999). We thus expect that attitudes toward smoking in pregnancy have changed, and that they are probably more negative and with less variability now compared than in the 1990s.
OBJECTIVES
Our research objectives were multiple: Firstly, we were interested in the percentage of smoking women at term and after childbirth, to gain better insights into the epidemiological situation in Switzerland and complement international data. This descriptive part fills a gap in the literature, as no recent data on our population of interest (at term pregnant women) are available. We also asked questions on information obtained on tobacco-related risks and the source of this information to identify potential gaps or needs. Next, we assessed a range of socio-demographic variables and risk factors to establish their links with the smoking status at term. Finally, we measured motivational variables from the TPB framework, and tested the association with the intention to remain abstinent after childbirth, and depending on the smoking status at term. This analytical part of the paper is important because to the best of our knowledge, only three studies have applied TPB to smoking in relation to pregnancy (Ben Natan et al., 2010; De Wilde et al., 2017; Godin et al., 1992) and two of them to post-partum (Ben Natan et al., 2010; Godin et al., 1992), their limitations having been discussed above. Replicating and extending existing results allows us to gain insights into reliable behaviour change levers for future interventions. Use of the CIBER analysis (Crutzen & Peters, 2019) is especially useful in this regard, as it highlights which variable(s) should be targeted for behaviour change interventions.
PARTICIPANTS AND PROCEDURE
PROCEDURE
This study follows a prospective design. Participants were recruited in the waiting room of the pre-birth consultations (prenatal period from 37 to 42 weeks of pregnancy) at the public hospital (Geneva University Hospital). They could either fill a paper-and-pencil version of the questionnaire, or use a tablet to answer the online version of the questionnaire. The study obtained ethics approval from the Regional Research Ethics Committee (CCER).
The questionnaire started with the information sheet and consent. Then the participants were asked for their email address, mentioning that they would be contacted again in a few months for follow-up. Participants then answered general socio-demographics questions, and questions related to their smoking status. Those answering that they were currently smoking or having quit smoking for pregnancy were also asked questions on their beliefs about postpartum abstinence, while the respondents who never smoked were thanked for their participation before this part of the survey.
Smokers and ex-smokers were sent questionnaires at T2, T3 and one reminder for each period of research. In order to minimize the losses at follow-up, a prize-draw was advertised, with participants having the chance to win a voucher in a baby store.
PARTICIPANTS
This study included any women of any age, in the prenatal period from 37 to 42 weeks of pregnancy. Our two inclusion criteria were a minimum level of French (self-assessed by the participants), and having an email address to enter the prize draw and receive the follow-up surveys.
We were able to collect answers from 210 women between April and August 2016, among which two were excluded due to an incorrect email address, thus N = 208. Mean age of participants was 31.87 (SD = 5.27). The majority of subjects (82.7%) were in a relationship (married, common-law), while 14.9% were single, and 2.4% divorced or separated. Half of the sample were expecting their first child, while the other half had already one or more children. 78.8% reported that the pregnancy was planned. The average income was between 52,000 and 104,000 CHF (40.4%), with 29.8% reporting earning more than 104,001, 11.5% 26,000 or below, and 16.3% between 26,001 and 52,000. Thus, the sample was rather diverse in socio-economic status. The flow diagram is presented in Figure 1.
MEASURES
Demographics. In the initial survey (T1), professional status, educational level, annual income, age and nationality were recorded. In addition, we asked respondents questions related to their pregnancy: civil status, number of children in total, whether the current pregnancy was planned or not, how many children were expected (one vs. twins or more).
Smoking status. We then asked the respondents if they were currently smoking (even occasionally). Those responding “yes” were then asked at what age they started smoking, and what their current consumption level was. All participants were asked if they were living with a smoker. If the answer was “yes”, an item asked if this person smoked in front of them inside the house, and what the consumption level of this person was. Finally, we asked all respondents if they had quitted smoking during the pregnancy or in anticipation of it. Those answering they never smoked were informed that the survey was over and thanked for their participation. Respondents saying “yes” to this question were then asked when they stopped smoking (response categories: before pregnancy / during the first trimester / during the second trimester / during the third trimester). Respondents saying “no” were asked if their consumption had changed during the pregnancy (response categories: yes, reduced / yes, increased / no, it didn’t change). If the answer indicated an evolution in the consumption, the question of the timing was asked (response categories: during the first trimester / during the second trimester / during the third trimester). All respondents at this stage were asked if they attempted to quit during the year before the pregnancy. They were also asked if they received information on tobacco-related risks during pregnancy. If they answered “yes”, details were recorded on the sources of that information (response categories: perinatal practitioners [doctor, midwife] / other health practitioner / family-friends / myself [own search for information]).
TPB predictors of motivation toward post-partum abstinence. We used direct measurement of TPB constructs (Francis et al., 2004). Three items for each construct of the TPB were presented (scale ranging from opposite bipolar adjectives [e.g. 1 – very difficult, 7 –very easy], or Likert scale ranging from 1 [totally disagree] to 7 [totally agree]), plus the possibility to answer “I don’t know”). Examples of items: “Not smoking during at least six months after giving birth is a good thing” (attitude); “My family and my friends think it is important that I do not smoke during at least six months after giving birth” (subjective norm); “Not smoking during at least six months after giving birth is: very difficult – very easy” (perceived behavioural control); “I am ready to make all necessary efforts not to smoke during at least six months after giving birth” (intention). Reliability of the items was satisfactory (attitude: α = .82, subjective norm: α = .83, perceived behavioural control: α = .89, intention: α = .99).
Postpartum smoking status (T2 and T3). We assessed tobacco consumption two and six months after giving birth by asking the person if they were smoking (even occasionally). If they answered “yes”, the consumption level was recorded. Questions on breastfeeding, professional situation (e.g., maternal leave), presence of a smoker at home, identification primarily as a smoker or as a non-smoker, and difficulties related to the quitting attempts (gain weight, craving, sleeping disorders, intestinal problems, emotional difficulties) were also asked at T2 and T3, but not analysed due to the small number of respondents at those measurement times.
RESULTS
PROPORTION OF SMOKERS IN THE SAMPLE (T1)
Among the 208 respondents at T1, 26.9% answered they had stopped smoking during pregnancy or when planning to be pregnant; 20.7% answered they had not stop smoking during pregnancy or when planning to be pregnant, and 52.4% answered they had never smoked. To the question “do you currently smoke?”, 14.9% answered yes, and 85.1% answered no. Combining the answer to those two questions led to a few incoherent situations: people answering that they do smoke currently but also that they did stop in relation to pregnancy, and people answering they do smoke currently to one question, but that they had never smoked to the other item. Those person were excluded from the sample (n = 10). We categorized the remaining sample (N = 198) into four groups: currently smoking (n = 21), smoking cessation because of pregnancy (n = 47), smoking cessation unrelated to pregnancy (n = 22), and never smoked (n = 108). Thus, in our sample, the percentage of at-term pregnant women smokers is 10.6%.
For the rest of the study we conducted the analysis by including only the persons saying they were currently smoking at term and those whose quit decision was related to pregnancy (total N = 68).
DESCRIPTIVE STATISTICS ON SMOKING STATUS AFTER CHILDBIRTH (T2 – 2 MONTHS AFTER CHILDBIRTH, T3 – 6 MONTHS AFTER CHILDBIRTH)
33 out of the 47 ex-smokers answered the survey at T2, and 78.8% of them remained non-smokers two months after childbirth, meaning that 21.2% relapsed. Concerning the women who were smokers at term, 15/21 answered the T2 questionnaire, and nearly all of them remained smokers two months after childbirth (93.3%).
Six months after childbirth (T3), 29 out of the 47 ex-smokers answered and 37.9% of them said they had started smoking again. Among the smokers, 100% of the T3 respondents (n = 11) were currently smoking.
DESCRIPTIVE STATISTICS ON SENSITIZATION ABOUT TOBACCO-RELATED RISKS DURING PREGNANCY (T1)
The majority of current smokers did receive information about tobacco-related risks (71.4%), while about half of ex-smokers did so (46.8%). Among those who received information, the most frequent sources were maternal-health professionals (doctors, midwife) (56.8%) and the person herself (personal search for information, web, public health information) (59.5%). The relatives of the person were a less frequent source of information (24.3%), and as general-health professionals (8.1%).
ODDS RATIO RELATED TO THE SMOKING STATUS AT TERM (T1)
A logistic regression was conducted on women who quit smoking due to the pregnancy and those who were currently smoking. Results are presented in Table 1. No socio-demographic variable was significantly associated with likelihood of stopping smoking during pregnancy. Effect sizes suggest that women with higher levels of education, as well as women with higher levels of income, were more likely to stop smoking during their pregnancy, and that first-time mothers had three times higher probability to be smoking at term than not being a smoker, compared to non-first-time mothers.
Table 1
Logistic regression results of socio-demographic variables and smoking status at term
MOTIVATIONAL VARIABLES (T1)
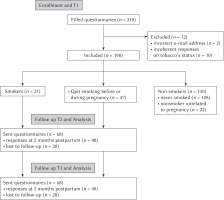
An analysis of the motivational variables predicting intention to remain abstinent after childbirth was conducted using Confidence Interval-Based Estimation of Relevance (CIBER) (Crutzen & Peters, 2019), implemented in the software Jamovi (The Jamovi Project, 2019).
An analysis of the plot (see Figure 2) reveals that all three determinants are highly correlated with intention to remain abstinent after childbirth. Attitudes scores are the most positive in the sample, meaning that most women have a positive attitude toward remaining abstinent after childbirth. The subjective norm score is more broadly distributed, suggesting variations in the perceived support from the close one toward remaining abstinent after childbirth. Finally, perceived behavioural control has a medium average level, and a broad distribution. This reveals that there is variety in how pregnant woman perceive the fact of remaining abstinent after childbirth; some perceive it as very difficult while others perceive it as effortless.
Figure 2
Confidence interval-based estimation of relevance plot for the TPB predictors (attitude, subjective norm, perceived behavioural control) on intention to remain abstinent after childbirth
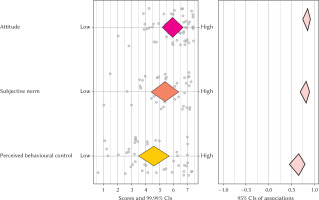
In Figure 3, the TPB scores are presented according to the smoking status at term. Unsurprisingly, women who stopped smoking for the pregnancy had in general higher scores on all variables than women who were currently smoking. Interesting differences can however be noted: the gap between the two sub-groups is wider for perceived behavioural control than for all other variables, with nearly no overlap.
Figure 3
Confidence interval-based estimation of relevance plot for the TPB predictors (attitude, subjective norm, perceived behavioural control, intention to remain abstinent after childbirth) associated with the current smoking status
Losses to follow-up prevented us from statistically testing differences in the measured variables between women who relapsed and those who remained abstinent postpartum. Descriptive statistics of those measures according to the postpartum status are provided in Table 2.
Table 2
Means and standard deviation of measured variables according to postpartum smoking status
DISCUSSION
This study aimed to provide a descriptive picture of the epidemiological situation of tobacco consumption among pregnant women at term and during the postpartum period up to 6 months after the child’s birth, and identify motivational levers for intervention.
One key result of this paper is that among the at-term pregnant women we surveyed for this study, 10.6% were still smoking at the end of their pregnancy (a number slightly higher than the national statistics, 7%). The relapse for those who quit smoking during (or for) the pregnancy ranges from 21.2% two months after giving birth, to 37.9% six months after the child was born. However, an important loss to follow-up could in fact mask a higher rate, as missing data are often not at random. For example, it was found that there was a link between the success of the quitting attempt and the probability of answering at the next measurement time (Desrichard et al., 2020). Applying the worst-case scenario to our data (i.e. that all the women who did not answer at follow-up had relapsed), the rate would rise to around 60% at T2, and 74% at T3. While this scenario might be overestimating the number of relapses (notably because after child birth there are a few reasons other than relapsing that would justify not answering the follow-up surveys), it suggests that the real percentage of relapse at post-partum is considerable. In terms of practical implications, this highlights the need to maintain the efforts for tackling this issue, given the high risks for the child’s health. Specifically, creation of consultation for pregnant women and training midwives in addiction support are necessary. In addition, because pregnancy is often a window of opportunity to intervene with women and their families (WHO, 2013), including for second-hand smoke (SHS) exposure, future studies could examine in more detail the circumstances of the first relapse cigarette, including the impact of women’s exposure to SHS. Exposure to SHS is a predictor of less smoking cessation during pregnancy (Riaz et al., 2018) and it is also a crucial health issue to the extent that, consistent with previous meta-analyses, Parascandola et al. (2019) found a significant increase in odds of low birth-weight, preterm birth, and stillbirth associated with SHS exposure in samples from high as well as for middle incomes countries.
The second key result of the study comes from the CIBER analysis, which suggests that perceived behavioural control is an important variable to consider, confirming results in the literature (Germeroth et al., 2019; Godin et al., 1992). All three TPB variables were associated with intention to remain abstinent during the postpartum period, but what the CIBER analysis reveals is levers for intervention. Indeed, contrary to the attitude which was very supportive of remaining abstinent after birth among most of the participants, the level of perceived behavioural control is more dispersed among our sample. This means that even if attitude is associated with intention, there is not enough variability in attitude for behaviour change intervention to try to change attitudes of pregnant women. In addition, and perhaps not surprisingly, a large difference was observed between the level of perceived control among at-term smokers and women who decided to quit because of pregnancy. What is not trivial is that there is no such large difference for the other TPB variables, meaning that women who were still smoking after childbirth and those who had quit during pregnancy had more or less the same perception of subjective norm, attitude and intention of not smoking after birth. Recommendations for practice would thus be that interventions targeted at pregnant women should focus on increasing perceived behavioural control. According to the Theory and Technique Tool (Human Behaviour Change Project, 2018), techniques to increase perceived behavioural control are notably: graded tasks, focus on past success, problem solving and instruction on how to perform a behaviour, the latter two being specifically identified by Brown et al. (2019) as effective to prevent postpartum relapse.
In terms of sociodemographic predictors, higher level of education and of income were marginally associated with higher chances of stopping smoking before term. This association was also found in previous research (El-Khoury et al., 2017; Riaz et al., 2018). Other variables which have been found in the literature to be predictors of smoking cessation during pregnancy were not found to be significant in our study. The small sample size, considered as a limiting factor in this aspect, can explain this difference. Interestingly, Riaz et al. (2018) found that nulliparous women had higher odds to quit smoking during pregnancy, while the relationship in our results was inverse (but not statistically significant).
Aside from the small sample size in the follow-up measurement, there are other limitations to be mentioned. We used a self-report measure of tobacco consumption, which could be subject to bias. Given the stigma associated with tobacco consumption, it is possible that participants minimized it. Similarly to what was discussed above about non-random attrition, the bias would be in the direction of an underestimation of tobacco consumption. A meta-analysis (Patrick et al., 1994) showed that the sensitivity and specificity of self-reports were accurate compared to biochemical measures, but self-administered questionnaires were less so than interviewer-administered questionnaires (5.2% points difference in sensitivity, 2.5% in specificity). Wray et al. (2016) found that biological measures were highly correlated with self-report in a sample of non-daily smokers – rs ranging from .39 to .75 depending on the type of self-report (e.g. daily report, graduated frequency) and the type of biomarkers (e.g. hair cotinine, carbon monoxide). Another limitation is the fact that pregnant women who do not speak French and do not possess an email address were not able to take part in the study. It is possible that these inclusion criteria might have excluded vulnerable persons, who are more at risk for tobacco consumption (Loring, 2014). Schaap et al.’s (2009) results show that this difference can range from 10% to 20% more ever-smokers in less educated women compared to more educated women in northern and western European countries. Not including those participants might then have resulted in an underestimation of tobacco consumption. This necessarily questions the external validity of the results, which is a recurring problem found in a large body of research on health prevention and interventions, particularly in the USA, where it has been shown that ethnic minority groups are under-represented in trials (Yancey et al., 2006) generally because of inclusion criteria. Including women speaking a wider range of foreign languages and changing the way women are recruited and data collected could optimize external validity and produce results applicable to all groups in the target population.
CONCLUSIONS
Data provided by this study suggest that the issue of smoking during pregnancy and post-partum relapse deserves attention. Incidence of tobacco consumption in our sample is non-negligible: one pregnant women out of ten is smoking at term, and will remain a smoker six months after childbirth. Post-partum consumption is also a risk for those who decided to quit because of pregnancy: four out of ten (and up to seven out of ten in the worst-case scenario) relapsed. Identifying smoking pregnant women in the early prenatal period should be promoted, and interventions that focus on increasing perceived behavioural control as a lever of change are promising.