Introduction
Immunoglobulin E (IgE) is considered the main component of type 1 hypersensitivity reaction [1] that clinically presents with anaphylaxis, asthma, atopic dermatitis, and allergic rhinitis [2, 3]. IgE-mediated food allergy is a common disease especially in children, with high-risk of potentially life-threatening anaphylactic reactions. A better understanding of the disease and active investigation for efficient prevention or treatment is required and important [4].
Primary immunodeficiency diseases (PIDDs) that usually result from inherent defects in the immune system, appear mainly with recurrent or severe infections but also, they may involve autoimmune disease, lymphoproliferation, or allergy [5]. Allergic diseases are an important expression of misdirected immunity, and certain PIDDs are frequently associated with atopy. Selective IgA deficiency (sIgAD) is associated with atopy, e.g. asthma, allergic rhinitis, atopic dermatitis, and food allergy [6]. And nearly 25% of patients with IgA deficiency are identified during evaluation for allergic disorders [7]. However, about 85-90% of patients with IgA deficiency are asymptomatic [8].
Secretory IgA plays an important role in the immune homeostasis of gut, as it provides first line defense through the aggregation, immobilization, and neutralization of pathogenic microbes and harmful molecules, which interact with mucosal surfaces [9]. Food proteins are one of the molecules that reach human mucosal tissues. Defective antigen elimination, because of dysregulation of the immune response in IgA deficient individuals, might be considered as a risk factor accompanying development of food allergy [10].
Indeed, many cross-sectional studies have shown a higher prevalence of atopic conditions either in patients with sIgAD [11] or common variable immunodeficiency, suggesting a potential association [12]. However, there are little definitive data in the literature on the overall prevalence of food allergies as a specific expression of clinically relevant atopy in patients with asymptomatic sIgAD. For this purpose, this work presents one of a few broad considerations of the prevalence of sIgAD among patients with food allergy.
Material and methods
This prospective case control study with analysis of clinical and immunologic characteristic of patients with food allergy referred to allergy and immunology outpatient clinic, Ain Shams University Hospital, which is one of the referral tertiary medical center in Cairo, Egypt. One hundred patients were recruited from January 2015 to March 2016. None of them reported recurrent episodes of respiratory tract infections. Patients with associated autoimmune diseases were excluded. Fifty healthy volunteers were selected as the control group.
Patients were suspected for diagnosis of food allergy based on a history of significant skin, digestive, or respiratory reaction related to exposure to any potential food allergen. Furthermore, foods suspected based on the history and skin prick test were completely excluded from the diet. Resolution of symptoms on elimination of offending foods and recurrence of symptoms by open food challenge test was suggestive of food allergy.
The allergy status of patients was evaluated by skin prick test on the forearm, using common standard allergen extracts (Stallergen, France), such as cow’s milk, wheat, soya, peanut, nuts, egg, fish, fruits, and other environmental antigens. Histamine and normal saline were simultaneously used as positive and negative controls, respectively. A wheal formation ≥ 3 mm above negative control after 15-20 min was considered as positive.
The study protocol was approved by the local research ethics committee of Ain Shams University, and informed consent was obtained from every participant included into the study.
Diagnosis of sIgAD was based on low or absent serum IgA level less than 0.07 g/l (< 70 µg/ml), and normal IgG and IgM levels in patients older than 4 years old [13]. Concentrations of IgA in plasma were measured by ELISA (Assaypro LLC, St. Charles, USA). A polyclonal antibody specific for human IgA was pre-coated on a 96-well microplate with removable strips. IgA in standards and samples was sandwiched by immobilized antibody and biotinylated polyclonal antibody specific for IgA, which were recognized by a streptavidin-peroxidase conjugate. All unbound material was washed away, and a peroxidase enzyme substrate was added. The color development was interrupted, and the intensity of color was measured. Normal serum IgA levels were 70-400 µg/ml.
Total IgE level was determined using Immuno CAP system (Pharmacia AB Diagnostics, Uppsala, Sweden), and the test was defined as positive for a value > 100 IU/ml.
Statistical analysis
Data analysis was performed by SPSS statistical software package, version 22 (SPSS Inc., Armonk, NY, USA). Data were expressed as mean ±standard deviation (SD) for parametric data, and as median and interquartile range (IQR) for non-parametric data. Parametric data were analyzed using Student’s t-test while non-parametric data were evaluated with Mann-Whitney U test to compare two groups. χ2 test was applied to compare categorical data, and Spearman’s correlation coefficient was performed to assess the strength of the relationship between serum IgA levels and different quantitative parameters. The statistical significance level was set as p = 0.05.
Results
One hundred patients with food allergy (62 males, 38 females), aged 28.6 ±7.4 years, were evaluated. Of the 50 healthy control, 74% were males, and their mean age was 30.1 years. The total serum IgE levels compared to control group was higher in 75 (75%) patients with food allergy. Characteristics of the study subjects are summarized in Table 1.
Table 1
Characteristic of the study groups
The most prevalent presentations of food allergy were urticaria/rash/eczema occurring in sixty-one subjects (61%), followed by asthma in thirty-six cases (36%), gastrointestinal manifestations (diarrhea or vomiting) in twenty-eight patients (28%), and hay fever in twenty-four (24%) cases.
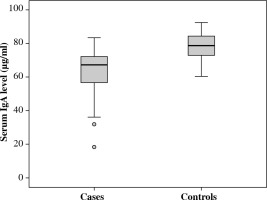
Skin prick tests for milk, wheat, egg, fruits, and nuts were predictive in almost all cases with positive reactions. IgA tended to low-normal range or below, and 67% of patients had serum IgA < 70 µg/ml. The median IgA in patients with food allergy was 67.3 µg/ml, and in the control group it was 78.6 µg/ml with only 2% diagnosed as cases of IgA deficiency (OR, 23.3; 95% CI: 8.1-66.06; p < 0.001) (Fig. 1).
Fig. 1
Serum IgA levels among cases with food allergy and controls. Values given are the median and interquartile range of serum IgA
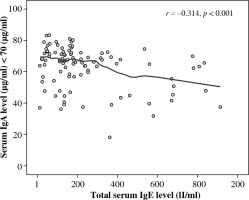
Furthermore, of 67 (67%) food allergic patients with asymptomatic IgA deficiency, only 53 (70.7%) demonstrated high levels of serum total IgE. As shown in Figure 2, serum total IgE among food allergic patients correlated significantly with serum IgA levels (r = –0.314, p = 0.001).
Fig. 2
Correlation between serum IgA and total serum IgE levels among food allergic patients (normal serum IgA < 70 μg/ml)
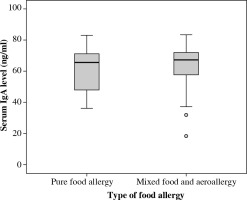
According to skin prick results, patients were further classified into two groups: group with pure food allergy and another group with combined food and aeroallergens. However, we did not detect any difference in levels of serum IgA between either of the two groups (OR, 1.41; 95% CI: 0.6-3.24; p = 0.430) (Table 2, Fig. 3).
Table 2
Serum total IgE, serum IgA, and other parameters among cases with food allergy and cases with combined food and aeroallergens
Discussion
Antibody production defects may be associated with health problems beyond just recurrent infection due to hypogammaglobulinemia but present an increased risk of allergy to alimentary antigens.
IgA deficiency prevalence is variable as most of cases are asymptomatic, and there is no established routine screening program for assessing IgA deficiency [7].
It has been suggested that 25% of patients with IgA deficiency could be identified during an allergy evaluation [7]. It is worth to mention that the prevalence of selective IgA deficiency among adult patients with food allergy in the current study was 67%, and the median of serum IgA was 67.3 µg/ml. This findings is consistent to those of Latcham et al., who reported that IgA tended to be at the low-normal range or below in food allergic patients (0.6 g/l), and 45% of children had IgA level below 0.3 g/l [14].
In a Korean study evaluating the relationships between serum IgA and allergic diseases in Korean children [15], serum values of IgA were much lower, with 31.1 mg/ml (range, 14.3-50.6 mg/ml) in cases of food allergy. This discrepancy may be attributed to the difference in age in the studied group and geographical distribution, as far Asian region has low prevalence (1/31, 800), which may suggest a genetic influence on the development of IgA deficiency [16].
Most previous studies assessed the prevalence of different atopic conditions among patients with sIgAD. In 1987, in his epidemiological study on immunoglobulin A deficiency, Klemola described a possibility of concomitant occurrence of allergic diseases and hypogammaglobulinemia in children and reported symptoms of atopic diseases in 50% of children with sIgAD [17]. This is practically similar to findings from a study by Szczawińska-Popłonyk et al. on food allergy in children with hypogammaglobulinemia indicating nearly 74% of children with symptoms of atopic diseases [10]. Despite low number of patients in a study performed by a research consortium known as the US Immunodeficiency Network (USIDNET), food allergy was found in 25% of those with sIgAD, which is similar to the results (30%) of Aghamohammadi et al. in a prospective cohort of Iranian patients with sIgAD and self-reported food allergy [18].
These above-mentioned findings are consistent with the current knowledge on mucosal secretory IgA involvement in the gut epithelial barrier function and immunological homeostasis, including antibody-mediated immune exclusion of microbial components [19] and tolerance mechanisms to foods [20]. Additionally, it has been demonstrated that both serum antigen-specific IgA and IgG antibodies possibly play an important role in protection against severe IgE-mediated food allergy. Therefore, the decrease of serum neutralizing IgG and IgA antibody levels that occurs in patients with hypogammaglobulinemia may predispose to increased intestinal mucosal permeability and subsequently, systemic absorption of ingested antigens, thus posing the risk of severe food allergy [10].
Moreover, an atopic patient might be at high-risk of systemic IgE-mediated reactions to alimentary allergens and in our study group, increased levels of serum total IgE were demonstrated in 70.7% patients with food allergy, in whom asymptomatic selective IgA deficiency was accidently discovered. This is consistent with a study by Tuano et al., who assumed that patients with sIgA deficiency have a higher incidence of pure IgE-mediated rather than mixed or non-IgE-mediated manifestations of disease [21]. However, in a Turkish study, serum IgE level increased by 37.3% in patients with IgA deficiency. On the contrary, in a Polish study on children with hypogammaglobulinemia, atopy in form of increased levels of serum total IgE was revealed in 8 of 17 children (47%) with food allergy [10]. Also, in another study by Dorsay and Orange, who retrospectively reviewed a group of 24 children with transient hypogammaglobulinemia of infancy, despite elevated levels of IgE in only 7 patients, twenty patients carried at least one atopic disorder [22]. These above-mentioned findings are supported by other authors’ opinions that patients with hypogammaglobulinemia and concomitant allergic diseases may demonstrate poor correlation between clinical symptoms and results of either serum total or allergen specific IgE tests. Therefore, serum IgE levels cannot be considered as suitable diagnostic criteria for allergic disease in patients with defective antibody synthesis [23].
Furthermore, in the present study, food allergic patients were classified into two groups based on existence or absence of concomitant aeroallergen. However, no difference in the deficient state of IgA was associated with either food allergy alone, or in combination with aeroallergen sensitization.
Conclusions
In conclusion, measurement of serum IgA should not be considered only in patients with recurrent infections but also should be suspected in cases of allergic and autoimmune diseases. Food allergic sensitization are associated with an increased prevalence of low-normal concentrations of plasma IgA. Dysregulation of the immune response contributing to defect in antigen elimination in predisposed immunodeficient individuals might be considered as a critical risk factor accompanying development of allergy.