Introduction
The diagnostic and therapeutic approach to axillary lymph nodes has long been considered indispensable in the treatment of breast cancer (BC) patients [1–3]. The importance of sentinel lymph node (SLN) detection regarding the accurate staging and prognosis in BC is immediately obvious. Until recently, SLN mapping of the axilla with the combined use of blue dye and radionuclides was the modality of choice. However, adverse effects of the injected dye, concerning mainly anaphylactoid reactions (about 1% of the patients) and permanent skin pigmentation, which affects 3.2% of the treated patients, have led practitioners to move towards more delicate methods [4]. Despite numerous techniques for sentinel lymph node mapping currently being in existence, there is a lack of recommendations for any specific mapping techniques that have been proven to be most effective. Therefore, evaluation of newly emerging mapping techniques that incorporate novel nodal visualization is crucial in ensuring better prognosis for BC patients. One such technique is the intraoperative visualization of lymph nodes, by incorporating real time three-dimensional (3D) field imaging via a multimodal system, called free-hand single-photon emission computed tomography (fhSPECT). This approach combines the previously used handheld gamma (γ-) probe, with a two-point tracking system, and an infrared tracking camera capable of localizing and displaying the tracer distribution with a portable, small γ-camera [5, 6]. Until now, the use of fhSPECT has been documented in various oncological procedures, with few reports on axillary staging [6–12]. Existing literature describing the use of fhSPECT showcases superior lymphatic mapping results compared to lymphoscintigraphy in melanoma patients [6]. The mapping capabilities of the modality are also documented in the intraoperative localization of parathyroid adenomas in smaller-scale publications, with encouraging results nevertheless [7]. Previous comparisons with the gamma probe technique in smaller scale studies also revealed superiority of fhSPECT in terms of detection rates [5, 8]. Researchers have also identified the potential of fhSPECT to reduce false-negative rates (FNRs), provided that the scans are of good quality [12].
Aim
Our retrospective study aimed to investigate the utility of fhSPECT and compare it with the intraoperative use of the gamma probe alone, in terms of average number of nodes detected per patient and length of the SLNB procedure.
Material and methods
For the purposes of this study, we retrospectively examined data from patients treated in our institution from November 2015 until April 2021. The protocol of our study was approved by the Bioethics Committee of our hospital (approval number 14550/9-6-2018). In order to facilitate data collection and handling, we set our patient recruitment policy at 50 consecutive patients for the gamma probe subgroup and 150 consecutive patients for the fhSPECT subgroup. The inclusion criteria were as follows: 1) core biopsy-confirmed breast neoplasm (either BC or high-grade DCIS) and 2) clinically negative nodal status at the time of diagnosis. We collected intra-operative and histological data from our patients, who were divided into two groups according to the SLN mapping technique used: The first group included patients where intraoperative radio-guided SLN nuclear detection was accomplished with the use of a handheld γ-probe, after periareolar Tc-99m-nanocolloid injection. In this group, SPECT/CT scanning was performed in the first 17 patients preoperatively. The second group included patients where the SLN detection was performed with intraoperative utilization of the freehand SPECT system. Intraoperative detection by the fhSPECT system was performed by the operating surgeon, under the assistance of nuclear medicine specialists. Patient records retrieved from November 2015 until January 2017 included patients who were treated utilizing the gamma probe alone. The fhSPECT system was employed for all of the patients within this cohort, consecutively from 2017 until 2021, as the standard of care, due to a change of treatment policies within our institution. The device employed was the declipse SPECT Imaging System (SurgicEye GmbH, Germany) (Photo 1). The use of the SPECT/CT system was gradually abandoned in favor of the fhSPECT system, taking into consideration patient discomfort, unnecessary delays and opting for the real-time imaging during surgery that the portability of fhSPECT actually added. Our operating team discontinued the use of the SPECT/CT imaging method after the results of a previous, intra-institutional audit that did not reveal any added value of SPECT/CT on intraoperative node mapping, when added to the gamma probe technique.
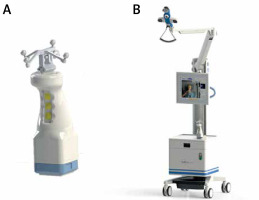
Photo 1
The declipse SPECT Imaging System. A – The high-resolution handheld Imaging Probe coupled with the tracking spheres. B – Tracking system and processing unit of the fhSPECT apparatus (Courtesy of SurgicEye GmbH)
A comparative analysis was done on the number of lymph nodes detected, the number of positive lymph nodes and the type of surgery (mastectomy or breast-conserving surgery, BCS). We opted for mean number of nodes detected as a means of comparing mapping methods, since it is indicative of the capabilities of each modality to scan and recognize lymphatic pathways. In addition, this measure has been used in previous publications regarding fhSPECT in SLNB [5–8]. The patients who received primary systemic therapy (PST) prior to SLN mapping were also recorded. The demographics of the study population are presented in Table I. A same-day injection protocol was performed in line with the current guidelines, regarding SLN localization in breast cancer [13]. Four periareolar injections of Tc-99m labeled nano-colloid (nano-sized albumin colloidal particles < 80 nm, Nantop, ROTOP) were administered approximately 45 min prior to surgery. Approximately 50 MBq (0.0013 Ci) were injected per patient. The pre-incisional acquisition scan followed. The acquisition scan consisted of lateral, anteroposterior and caudocranial views, lasting approximately 4 min, defining the rectangular volume of interest (VOI) – a three-dimensional virtual cubic space within which the SPECT camera system was to scan for emitting hotspots (Photo 2). This was done in a way similar to the protocol proposed by Wendler et al. [12]. The γ-probe was placed just above the skin level, with a cranial direction. Meanwhile, a continuous scanning motion with a speed of 1 cm/s was applied throughout the surgical field. The surgeon (M.A., one of the authors) proceeded with the incision of the overlying skin and adipose tissue at a depth suggested by the probe’s indications. The probe was then inserted into the surgical field, in order to perform the first intra-field scan. The system’s depiction of the distribution of radionuclide inside the surgical field is seen in Photo 2. Retrieval of “hot” nodes followed, adhering to the recommendation of removing any node with an isotope emission reading > 10% of the node with the highest reading. One example of the system’s monitor indication's is given in Photos 3–5, as taken from some of our cases. After completion of the whole process, the surgical field was rescanned to confirm that no radioactivity was left in place.
Table I
Patient demographics
Photo 2
The surgical field imaging, as taken from the processing unit. The system’s VOI, detected hotspots, distance from target, as well as both tracking devices are visible
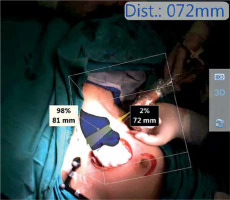
Photo 3
Intra-field reconstruction of the system. The targeted lymph node, emission rate, as well as the distance from the target can be clearly seen
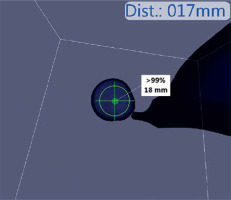
Photo 4
A–F – Serial scanning of a single lymph node, from multiple directions, performing the “pivoting scanning motion”. More details are given in the text
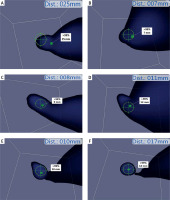
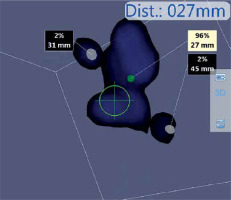
Photo 5
A nodal cluster, encountered during the SLNB process. Emission measurements and distance from probe for each of the hotspots can be seen
Statistical analysis
Following data acquisition and structuring, a statistical analysis was performed. We utilized Student’s t-test and the Mann-Whitney U test, for comparison of numerical datasets and deduction of statistical significance. The Mann-Whitney U test was employed in average node per axilla comparison, since data analysis with the Kolmogorov-Smirnov test indicated a lack of normal distribution, thus not achieving the required assumptions for the t-test. Pearson’s χ2 test was applied to compare the statistical significance of percentile differences between the two groups. Statistical significance was considered for p < 0.05.
Results
In total, 150 patients with a mean age of 62.6 ±12.9 (range: 34–91) years were included in the fhSPECT group and 50 patients with a mean age of 62.8 ±11.2 (range: 38–84) years in the γ-probe group. Histological grade varied between the two groups, as grade I carcinomas made up 21/50 (42.0%) versus 24/150 (16.1%) (p = 0.0002) in the γ-probe and fhSPECT group, respectively. Grade III carcinomas also differed: 6/50 (12%) as opposed to 47/150 (31.5%), respectively (p = 0.006). When comparing the histological N0 nodal status after SLN mapping, there was a statistically significant difference between the γ-probe and fhSPECT group, with 41/50 patients (82.0%), and 90/150 (60.4%), respectively (p = 0.006). N1mi nodal status also differed, with 0/50 and 13/150 (8.7%), respectively. The percentage of cancer in filtered lymph nodes of those excised also varied significantly between the two groups with 15/183 (8.2%) in the γ-probe group, versus 116/623 (18.6%) in the fhSPECT group (p = 0.0008). In 8.66% of the patients in the fhSPECT group, level II and apical LNs were detected. This is a novel finding, not observed under the γ-probe. SLNB was converted to axillary lymph node dissection (ALND) in 3/50 (6.0%) in the γ-probe group compared to 18/150 (12.0%) in the fhSPECT group. Even though the percentage of conversion to ALND was doubled when using fhSPECT, statistical analysis failed to demonstrate any statistical significance (p = 0.231).
On the other hand, the mean number of nodes detected and excised differed significantly between the two groups. Utilizing the γ-probe device, a mean of 3.66 lymph nodes were excised per axilla, versus 4.18 LNs in the fhSPECT group (p = 0.03). In the gamma probe group, the average operation time was 39 ±7 min, compared to 37.54 ±17.11 min in the fhSPECT group (p = 0.23). However, comparison of the mean SLNB time between the gamma probe (n = 50) and the 2021 group of patients (n = 17) revealed a significant shortening of surgical times from 39 ±7 min in the former to 32.35 ±10.46 min in the latter (p = 0.010). With regard to the length of operation on an annual basis, a gradual reduction was recorded after fhSPECT implementation. The average annual operational time throughout the study period was significantly decreased from 40.2 ±20.77 min in the 2019 group of patients (n = 38) to 32.35 ±10.46 min in the 2021 group of patients (n = 17) (p = 0.033). Both these measurements indicate a statistically significant trend in reduction of the surgical time. The above findings regarding surgical times are summarized in Table II. A minimum of three lymph nodes per axilla were excised in 36/50 (72.0%) of the patients in the γ-probe group, compared to 123/150 (83.3%) in the fhSPECT group, a difference that for the current number of patients was not statistically significant (p = 0.107). However, the average number of excised lymph nodes per patient has higher in the fhSPECT group (4.16 vs. 3.66 in the γ-probe group, p = 0.03). Studying the subpopulation of obese patients in both groups revealed a small reduction in operational times from 45.5 ±3.09 min to 44.04 ±20.9 (p = 0.27). However, the number of excised lymph nodes per patient was higher on average in the obese patients of the fhSPECT group (4.26 ±1.44) than in the γ-probe group (3.2 ±1.65) (p = 0.043) following closely the cumulative results. A summary of our findings can be found in Table III.
Table II
Study results. Comparison of surgical times
Table III
Study results. Comparison between γ-probe and fhSPECT
Discussion
Our study showed that the fhSPECT method is more efficient than the gamma probe method in LN detection (as expressed by mean LNs per patient), an observation that also holds true for obese patients. We also found that there is a good potential for reducing surgical times of the SLNB operation. A minimum of three lymph nodes was detected in significantly more patients within the fhSPECT cohort. In this study, we investigated a promising technique for SLN mapping and biopsy. To the best of our knowledge, this cohort is the largest regarding the use of fhSPECT in SLN biopsy for breast cancer. Regarding breast cancer SLN mapping, intraoperative use of a radioisotope tracer has long been used as the method of choice. The addition of a colorimetric modality in the form of blue dye is currently the gold standard, with detection rates of up to 96.5% [14–17]. However, use of the dye technique has been associated with a wide range of adverse effects [18]. Currently, there is a growing need for the development of sophisticated mapping techniques that would be safe, cost-effective, and could reduce surgical times while improving the LN detection rates. Wendler et al. first introduced fhSPECT as a novel technique for lymphatic mapping, while SPECT/CT was used as the validation method [12]. Their results showed a detection rate of 77.8% in their pilot study, and 83.3% in their validation study, indicating relatively low accuracy, especially when considering that only good quality scans had a detection rate of only 77.8% in the pilot study. Of note, the number of patients with only one SLN (46/85 patients) detected was considered high, as was the number of patients with no SLNs excised. In our study, at least 3 LNs were identified in most patients, and few pathological reports confirmed less than 2 LNs. Our study showed that a minimum of 3 LNs were detected and excised in 129/150 (83.3%) of the patients in the fhSPECT group, compared to 36/50 (72%) of the patients undergoing SLNB utilizing γ-probe detection alone. We opted for a minimum removal of 3 SLNs per patient, in accordance with reliable published data, which show that 97–98% of positive SLNs are found within the first three excised lymph nodes [19]. Use of fhSPECT has a unique advantage over other mapping methods: the virtual optical information provided during the operation. The system’s “almost real time” projection of the reconstructed field image, with the addition of depiction of radioactivity distribution within the axilla, is a complementary guidance tool that promises favorable outcomes, regarding the precision and feasibility of SLNB.
Expanding on the use of the fhSPECT method on breast cancer, a study by Bluemel et al. compared preoperative scintigraphy and intraoperative use of both the γ-probe and fhSPECT [5]. Using scintigraphy as a gold standard, the fhSPECT system surpassed the γ-probe in detection rates (92.3% and 89.7% per axilla, respectively), results that are consistent with our findings. In our study, fhSPECT detected an average of 4.2 (SD 1.78) SLNs per axilla, while the gamma probe detected 3.48 ±1.63. Similar to our results, Bluemel’s study also found the use of fhSPECT to vastly outperform the γ-probe in SLN detection rate (92.7% vs. 69.1%). It ought to be noted, however, that despite the statistical significance of our findings, it seems largely unclear whether an observed difference of 0.72 nodes on average can have any significant clinical impact, since one cannot retrieve less than one lymph node. Our findings clearly indicate a significant trend towards superior nodal detection of the fhSPECT system compared to the gamma probe that is still in need of better evaluation through larger patient groups.
FNRs in SLNB have been estimated to be 8–10% [17, 20]. The system’s sensitivity compared to planar scintigraphy was reported at 97.5% [21]. Reports on the factors associated with higher FNRs indicate that the number of excised SLNs is an independent factor that negatively affects FNRs [22]. Therefore, we can argue that implementing novel mapping systems such as fhSPECT, that are able to detect more SLNs on average, can lead to a reduction of FNRs. In our study, we could not address the matter of FNRs, mainly due to existing guidelines on the management of the axilla in early-stage BC patients, which no longer recommend performing ALND as a viable therapeutic or staging option. The “10% rule”, the proposed upper numerical limit of nodal excision, has been shown to often cause over-excision of nodes when they are closely adhered to [23]. Herein, we found that the use of fhSPECT not only leads to easier and faster node acquisition, but could even optimize the number of SLNs excised per axilla. FhSPECT also provides valuable assistance regarding the upper limit of total lymph nodes excised. In this aspect, fhSPECT, as compared with the γ-probe, offers the advantage of simultaneous dual indication (CPS and depth) for each node, reducing the FNRs. In addition, the range of the system’s intrinsic resolution capacity is 1–3 mm, making the distinction between two different lymph nodes far more accurate than with the γ-probe. To put matters into perspective, previous reports of similar systems with encouraging nodal detection results looked at systems with spatial resolution of 12–14 mm [6]. This capacity enabled us to identify and remove the true emitting node, without unnecessary dissection of the surrounding lymphatics. False negative rates are of particular interest in a specific subpopulation of patients, those having undergone primary systemic therapy (PST). In our study, 10 patients after PST were included in the fhSPECT group. In these patients, the average detection time was 34.8 ±18.14 min with a mean of 6.1 ±1.45 LNs detected. The small number of post-PST patients enrolled did not allow for comparison between the two groups. Post-PST inflammatory reaction of the axilla may lead to altered regional lymphatic drainage, making false negative detection far more frequent. A 2016 meta-analysis found that the false negative rate was 13% for patients having received PST [24]. Current guidelines recommend the use of dual-mapping technique for SLNB in post-PST patients, as a means of reducing the FNR [25]. Furthermore, any isolated tumor cell counts in the decision to perform ALND [26, 27]. Thus, a combined-mapping technique (such as fhSPECT combined with the γ-probe) seems appropriate for staging these patients. Three-dimensional (3D) reconstruction of radionuclide distribution of the surgical field enables the surgeon to obtain multidirectional imaging of the possible SL nodes, including the depth (Photo 4), and therefore aid in the optimal identification of SLNs, despite the extensive fibrosis seen in the axilla after PST. Thus, introduction of more sensitive techniques that could lower FNR, such as fhSPECT, would be of great value in the overall treatment of such patients. Due to the small number of patients, no comparison could be made between the groups. Furthermore, another point of interest in the utilization of fhSPECT is the detection of level II, including apical, sentinel lymph nodes. After the introduction of the fhSPECT system in our clinical practice, we noted an increase in the detection of level II lymph nodes, including apical ones. We recorded level II lymph nodes in 13/150 (8.66%) of the SLNBs when fhSPECT was used. Existing literature shows little experience in non-level I SLN mapping. In the original publication by Wendler et al., level II SLNs were found in 3 of the patients, while Bluemel et al. reported the detection of such nodes [5]. The ability of fhSPECT to map previously unseen deeper locations of lymph nodes will contribute to uncovering unusual and complex lymphatic drainage, making the disease staging more accurate.
Obesity, especially when combined with advanced age, has long been documented to negatively influence the success rate of SLNB and increase FNRs [28]. A study by Cox et al. showed that an increase of either 1 year of age, or 1 unit of BMI, lowered the SLNB success rate by approximately 5% [29]. The use of SPECT/CT was found to amplify the acquisition rate of SLN identification in such patients, when compared with use of lymphoscintigraphy and blue dye [30]. While our study was not able to demonstrate expedited nodal detection in obese patients, fhSPECT allowed for more nodes to be detected on average. Similar to previous findings, this is expected to reduce the false-negative SLNB operations in obese patients. In addition, there are qualitative advantages as well; the real-time spatial navigation of the axilla will undoubtedly facilitate nodal excision in patients with copious amounts of adipose tissue.
Surgical time is of major concern in SLNB, since a prolonged procedure could alter aTc-99m nanocolloid drainage. Here, we observed that the mean acquisition and retrieval times have been gradually reduced since the introduction of fhSPECT. It is noteworthy that the length of the first 3D scan has been found to be highly dependent on the experience of the operator with the system [31]. In our study, we observed a reduction of the overall length of the SLNB, which has progressed from a mean of 46 min to 34 min on average. This is proof that the use of fhSPECT system has a brief learning curve, and surgical times decrease as the operator’s experience grows.
One of the most significant and novel findings in our study was the progressive reduction of the repeat field scans needed, as the surgeon moved deeper in the tissue. This can be attributed to the scanning technique employed by the surgeon (M.A.), which utilized a “pivoting” scanning motion, in a way that essentially performed a brief rescan on multiple angles around the hotspot while moving deeper into the tissue, leading to the improvement of reconstruction and acquisition time. A demonstration of the “pivoting” scanning is seen in Photo 4. While performing the aforementioned scanning process, the concurrent utilization of the system’s depth estimation allows for better delineation of the LNs in the axillary space, by enabling the multangular depiction of hotspots, thus making the technique capable of distinguishing the target LNs among a cluster of emitting nodes, in order to selectively harvest the highest emitting ones. An example of clustered lymph nodes, as depicted by the system, can be seen in Photo 5. While no quantitative analysis could be performed on the scanning motions of the operator, this is a qualitative finding that demonstrates the importance of spatial navigation within the axilla. Future studies could evaluate different scanning protocols and determine whether there is any further surgical significance.
One of the limitations of this study is the relatively low number of patients included in the γ-probe study group, thus making the statistical correlation more difficult, while remaining relative. An ideal comparison between the two methods should be to employ both mapping techniques for the same patients simultaneously. This would also allow for better estimation of the FNR and accuracy of fhSPECT. However, this is deemed inappropriate due to unacceptable prolongation of surgical time, while adding unnecessary patient discomfort. The retrospective nature of our study is another limitation. In order to minimize bias and produce higher quality results in the future, research questions comparing mapping methods can use randomized patient cohorts instead. In addition, future studies on the subject could attempt to recruit specific subgroups of patients (e.g. obese, post-PST) to confirm the significance of our findings within a smaller scale patient cohort.
Conclusions
Being a unique combination of complementary mapping techniques (SPECT and γ-probe), the modality’s biggest advantage is the real-time 3D spatial reconstruction and depiction of the surgical field, a fact that, in essence, brings SPECT/CT lymphoscintigraphy into the operating room. This modality could be the answer to the growing need for more precise methods in SLNB and lower FNRs, by allowing for more LNs to be detected on average, as demonstrated by our findings. In addition, our results show a tangible decrease in surgical times, with only a modest learning curve, while remaining a safe and accurate option. The detection of the unexpected level II lymph nodes is another novel advantage of the method. Furthermore, its implementation as a mapping method in obese and, especially, post-PST patients promotes the fhSPECT method as a reliable alternative, with distinct advantages over other techniques, as seen by the reported shortening of the surgical times. The original report of the “pivoting” scanning motion employed here further enhances the mapping potential of this modality. In conclusion, we suggest that fhSPECT should be popularized and incorporated into the SLNB procedure whenever possible. It should be noted, however, that wider implementation of the fhSPECT system would also require the presence of nuclear medicine laboratories and specialists, a perquisite not met by many breast cancer centers.









