Sulodexide (SDX) is a heparinoid used in chronic venous and arterial diseases [1]. It is also proved that SDX protects against ischemia/reperfusion injury [2]. SDX possesses endothelial protective effects due to its antioxidant, anti-inflammatory and antiproteolytic properties [1]. In endothelium, SDX interacts with nuclear factor-erythroid-2-related factor (Nrf2)-antioxidant response element (ARE) and nuclear factor-kB (NF-kB) signaling pathways [1, 3]. Nrf2 regulates the expression of enzymes involved in glutathione (GSH) synthesis and utilization such as g-glutamylcysteine ligase (GCL), cysteine-glutamate exchanger (xCT), glutathione synthase (GS), glutathione peroxidase (GPx) and glutathione reductase (GR). ARE was found in the promoters of all these GSH-associated genes [4]. GSH, a key antioxidant, is synthesized in two ATP-dependent steps. The first step is rate limiting and determined by GCL activity and the intracellular level of cysteine. GCL catalyzes a coupling reaction between glutamate and cysteine, whose product is γ-glutamate-cysteine (γGC). GCL consists of a catalytic subunit (GCLc) and modifier subunit (GCLm) which are regulated independently at transcriptional and post-transcriptional levels. However, the transcriptional control of GLCc gene expression is the most important for GCL activity [5]. System xc – , a cystine/glutamate antiporter with xCT and 4F2hc subunits, facilitates the uptake of cystine, which is quickly reduced to cysteine for GSH synthesis. Transcriptional regulation of the xCT gene is controlled by the Nrf2-ARE pathway and xCT mRNA expression correlates with system xc – activity [6]. The second step of GSH synthesis is catalyzed by GS, which adds a glycine to gGC. GS expression is regulated only at the transcription level [4]. During oxidative stress, GPx catalyzes the reaction of hydro and lipid peroxides with GSH resulting in the formation of oxidized glutathione (GSSG). GPx1 is the most abundant intracellular isoform of GPx in human endothelium. GR then converts GSSG to GSH using NADPH [5]. GPx1 and GR are regulated mainly at the level of transcription [7].
In the present study, the variations in the transcript levels of GSH-related genes (GCLc, xCT, GS, GPx1 and GR) were evaluated in HUVECs subjected to oxygen-glucose deprivation (OGD) and treated with SDX.
Methods
HUVECs (Lonza, USA) were cultured in EGM-2 with EGM-2 BulletKit as previously described [3]. SDX (0.5 LRU/ml) was added to glucose- free DMEM and cells were harvested in a hypoxic chamber (3% O2, 5% CO2, 92% N2 at 37°C) after different OGD times (1–6 h). External control groups (normoxia) in EGM-2 with EGM-2 BulletKit were exposed to normoxia for 6 h. Internal controls (OGD) were incubated in OGD but not treated with SDX.
Total RNA was extracted using TRIzol reagent (Sigma, USA). A high-capacity cDNA reverse transcription kit (PE Applied Biosystems, USA) was used for cDNA synthesis. qRT-PCR was performed using SYBR Select Master Mix (PE Applied Biosystems) in the Roche LightCycler 480. The relative mRNA expression levels were normalized to GAPDH using the 2–ΔΔCt method. The primers of target genes were as follows: GCLc, 5′ TGTCACTGTTTTCACCATTCAA 3′ and 5′ GGACGAGGATGAGGAGGAG 3′; xCT, 5′ GTAGGCCACATTTGTCAGCA 3′ and 5′ GCT-GGCTGGTTTTACCTCAA 3′; GS, 5′ TTGAAGTCCATCTGCACAGC 3′ and 5′ CAAGC-TGGGTGGCACTTG 3′; GR, 5′ TTGGAAAGCCATAATCAGCA 3′ and 5′ CAAGCTGG-GTGGCACTTG 3′; GPx1, 5′ CGGGACTACACCCAGATGAA 3′ and 5′ TCTCTTCGTTCT-TGGCGTTC 3′; GAPDH, 5′ GAAGGTGAAGGTCGGAGTC 3′ and 5′ GAAGATGGTGATG-GGATTTC 3′.
Results are reported as mean ± SD. Statistical significance was determined using one-way ANOVA and Bonferroni’s test, with p < 0.05.
Results
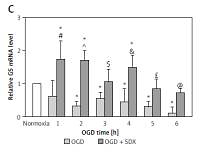
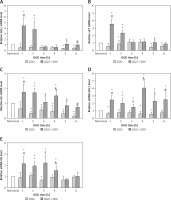
QRT-PCR was used to investigate the effects of SDX (0.5 LRU/ml) on expression of the Nrf2-driven GSH-related genes in OGD-injured HUVECs. This method allowed us to compare the effects of OGD alone and SDX in OGD by comparison with untreated (normoxic) cells, which were normalized to one. SDX dramatically increased mRNA levels of GSH biosynthesis genes, GCLc and xCT, in the first 2 h of OGD (Figures 1 A, B). Furthermore, the treatment of HUVECs with SDX for 1–6 h OGD caused a significant increase in GS and GPx1 mRNA expression levels (Figures 1 C, D). The transcript levels of GR, the GSH recycling gene, were significantly elevated after 1–4 h of incubation with SDX in OGD (Figure 1 E).
Figure 1
SDX induces expression of GSH-related genes. HUVECs were incubated in normoxia or OGD (1–6 h) without or with SDX (0.5 LRU/ml) and mRNA levels of GCLc (A), xCT (B), GS (C), GPx1 (D) and GR (E) were measured by qRT-PCR. Data are mean ± SD (n = 4)
*p < 0.05 vs. normoxia; #p < 0.05 vs. 1 h OGD; ^p < 0.05 vs. 2 h OGD; $p < 0.05 vs. 3 h OGD; &p < 0.05 vs. 4 h OGD; £p < 0.05 vs. 5 h OGD; @p < 0.05 vs. 6 h OGD.
Discussion
Vascular diseases related to oxidative stress, such as ischemia, are affected by endothelial dysfunction and redox imbalance with low intracellular levels of GSH [5]. Impairments in Nrf2/GSH-related genes/GSH cascades have been reported in ischemia-induced endothelial injury. Furthermore, several antioxidants (e.g. quercetin, resveratrol) have been shown to activate Nrf2 and up-regulate GSH-dependent genes with an increase in GSH levels in ischemic endothelial cells [5].
SDX protects against endothelial ischemic injury but the exact mechanism of its anti-ischemic action is not clear [2]. It is known that Nrf2 is an important regulator of GSH-related gene expression [8]. We recently reported that Nrf2 and glutathione S-transferase p1 (GSTP1) are up-regulated in response to SDX in HUVECs exposed to OGD [3]. Here we found that SDX induces very early and transient transcription of de novo GSH synthesis genes, including GCLc and xCT (Figures 1 A, B). Since both GCLc and xCT are linked to GSH production, these alterations could have an initial effect in increasing the GSH levels by SDX, resulting in cellular resistance to oxidative stress [9]. In our study, also transcriptional activation of GS and GR by SDX was observed in HUVECs exposed to OGD (Figures 1 C, E). There is evidence that in endothelial cells with a requirement for a large capacity to synthesize GSH it is necessary for the expression of GS and GR genes to be adequately elevated [10]. GSSG accumulation in cells upon ischemic stress is highly toxic and therefore GR is one of the most relevant factors involved in protection against oxidative damage. Furthermore, the time-course analysis shows that SDX induced a prolonged antioxidant response by a stronger GPx1 expression in later hours of OGD (Figure 1 D).
Hence, this study suggests that SDX acts as a strong protector of endothelial cells under ischemic injury. For clinical application to patients with ischemic vascular disease, this study has many limitations because of confirmation only by in vitro study. However, these findings may provide the basis for further experimental and clinical studies on the endothelial protective effects of SDX in ischemic events.
In conclusion, we report the novel finding that SDX induces GSH-related genes in human endothelial cells exposed to simulated in vitro ischemia. Our results provide further insights into antioxidant properties of SDX.