Introduction
The use of Aquafilling is often associated with various health complications, such as breast deformity, filler migration, mastalgia, palpable hypoechoic lumps, breast tenderness, breast abscess, breast fistula, and inflammation of the mammary glands. The main clinical concern associated with these risks is the effective removal of Aquafilling from all tissues that come into direct contact with the filler [1-6]. The filler is an inflammatory process triggered at the site of tissue contact. This raises concerns about the possibility of tumor formation at both direct injection sites and the site of product migration.
To evaluate the validity of this notion, we compared the immunohistochemical expression of E-cadherin and N-cadherin in patients who underwent surgical removal of Aquafilling from the breast, with patients after breast augmentation with implants or breast lifts. Lowered E-cadherin and simultaneously increased N-cadherin expression were previously associated with epithelial-mesenchymal transitions (EMT), a mechanism crucial in carcinogenesis. While more than 30 types of trans-membrane cadherin molecules are involved in this process, the effects of E-cadherin and N-cadherin are currently the best described [7-9].
However, the opinions of researchers regarding Aquafilling treatment are still inconclusive. Most of them consider filler removal from the injection site as an absolute necessity. However, there is no clear indication as to whether this treatment is sufficient or if subcutaneous mastectomy is required as a definitive solution to the problem. Total subcutaneous mastectomy takes away a woman’s ability to lactate, and many patients who have undergone Aquafilling treatment are still of reproductive age. It is also important to remember that breast fat is an endocrine organ that secretes e.g. adipokines, cytokines, chemokines, and growth factors [10]. A total of 359 unique proteins expressed by that tissue have been identified, controlling a broad range of cellular processes. These include signal transduction and cell communication, energy metabolism, protein metabolism, and the immune response. In addition, mammary adipose tissue is a reservoir of interstitial fluids that contain both locally secreted molecules and those assimilated from distal sites [11].
Proteomic analysis of adipose tissue and its interstitial fluid revealed the presence of signaling molecules, cytokines, hormones, and growth factors.
The main function of adipocytes is to store energy in the form of lipids. Hence, the detected proteins play a role in energy metabolism. Adipocytes show a pro-inflammatory effect by secreting cytokines (interleukin: IL-6, IL-8, IL-10, transforming growth factor [TGF], tumor necrosis factor [TNF], nerve growth factor). Factors enabling fat expansion have also been found. These include epidermal growth factor, fibroblast growth factors, hepatocyte growth factor, TGF, and leptin, as well as tissue metalloproteinase inhibitors (TIMP-1 and TIMP-2), which inhibit the activity of metalloproteinase. Moreover, they produce angiogenic factors (vascular endothelial growth factor, angiogenin, angiopoietin-2).
A very important mechanism occurring in adipose tissue is apoptosis, which plays an important role in both adipocyte development and homeostasis. The number of adipose tissue cells is regulated, at least in part, by an apoptotic signaling pathway involving caspase activation. In the study by Celis et al. they identified several components of the caspase cascade (caspase-3, -6, -7, -8, -10, -11) as well as others such as apoptosis-inducing factor, Smac/DIABLO, Bcl-x, and Bcl-10 and components of the death receptor signaling pathway (Fas and DAXX) [11].
There are also transcription factors (c-Jun, c-Myc, and E2F1) in adipose tissue, which are responsible for the adipogenesis process. The ubiquitin-like protein (NEDD8) has also been found to control the activity of the ubiquitin ligase complexes of the stem cell factor. Consequently, it may be a modifying factor for the suppressor proteins p53 and Mdm2 and lead to the formation of cancer [11].
Based on the above, which are only some of the proteins present in breast adipose tissue, it can be concluded that the biology of adipocytes is complex and multifaceted. Due to the lack of complete knowledge of the mechanisms occurring in the breast adipose tissue, any external interference may cause complications that are difficult to predict.
Inflammation is the immune system’s biological response to various factors that can induce an acute or chronic response. The inflammatory response is dependent on the type of inducing stimulus. However, regardless of the causative agent, it shares the following steps: recognition of the harmful agent by cell receptors, activation of inflammatory pathways, the release of inflammatory markers, and recruitment of inflammatory process cells [1, 12]. Inflammation damages the extracellular matrix and decreases cell adhesion [13].
In turn, cell-cell adhesion is a key component of epithelial tissue integrity and homeostasis during the response to various types of damage. Loss of cell adhesion and acquisition of mesenchymal features are part of a phenomenon known as epithelial-mesenchymal transition (EMT) [14]. It is observed in both physiological and pathological processes. Due to biochemical changes, cell-cell connections are lost, increasing their mobility. Consequently, they gain the ability to migrate. Key molecules involved in intercellular interactions include integrins, selectins, cadherins, trans-membrane proteins, and trans-membrane adhesion molecules of the immunoglobulin superfamily. Epithelial phenotype proteins of decreased levels are among the typical markers of EMT, such as E-cadherin. At the same time, as a result of mesenchymal cell feature acquisition, elevated N-cadherin activity, among others, is observed during EMT. This mechanism can occur during tissue regeneration and remodeling under the influence of inflammatory factors [15, 16].
N-cadherin is a calcium-dependent single-chain trans-membrane glycoprotein that also mediates cell adhesion. It plays an important role in the developmental and functional regulation of the nervous system, brain, heart, skeletal muscle, blood vessels, and hematopoietic microenvironment. However, abnormal expression of N-cadherin has been described in many malignancies such as lung cancer, breast cancer, prostate cancer, and squamous cell carcinoma. It is increasingly recognized that abnormal N-cadherin expression is closely associated with aspects of human malignant tumor progression such as transformation, adhesion, apoptosis, angiogenesis, invasion, and metastasis [17].
E-cadherin is a transmembrane glycoprotein that forms anchoring junctions by linking with the cadherins of neighboring cells, providing connection points between their cytoskeletons. The cytoplasmic ends of cadherins bind to catenins, which connect actin filaments to actin-binding proteins. Due to these properties, E-cadherin plays a role in inhibiting tumor formation. Hence, dysregulation of its expression leads to carcinogenesis [18].
This study aimed to semiquantitatively compare the immunohistochemical expression of E-cadherin and N-cadherin in tissue material from two groups of patients. The first group underwent surgical removal of Aquafilling from the breast, while the second was subjected to breast augmentation with implants or breast lifts.
Material and methods
Population
The study group consisted of tissue samples from 16 patients who had Aquafilling removed, while the control group comprised samples from 16 patients who underwent breast augmentation with implants or breast lifts.
The mean age of the women in the study group was: 33.6 ±6.69 years (mean ±SD), 36 (median), 21-46 (range). Furthermore, the time elapsed from injection of Aquafilling to its removal by surgery was: 43.4 ±16.7 months (mean ±SD), 38 (median), 12-81 (range).
The volume of Aquafilling filler applied to the breasts of women in the study group ranged from 100 ml to 260 ml.
Morphological and biochemical parameters (before operation)
Morphological parameters, namely leucocyte, erythrocyte, monocyte counts, and hemoglobin levels, were assessed in all patients.
Biochemical parameters, namely C-reactive protein (CRP), urea, creatinine level, activated partial thromboplastin time (APTT) and international normalized ratio (INR), were assessed in all patients.
Histological examination
The tissue material was prepared according to the standard histological procedure. Slides for histopathological evaluation were stained with hematoxylin and eosin.
Immunohistochemical studies
Immunohistochemical studies were performed according to the procedure indicated by the manufacturer – Vector Laboratories (user guide).
The 4 µm thick sections were deparaffinized and then rehydrated with xylene and a series of alcohols. Antigens were retrieved at 96°C for 20 min, in Vector Antigen Unmasking Solution, Citrate-based, pH 6.0 (H-3300). Endogenous peroxidase activity was blocked in BLOXALL blocking solution for 10 minutes.
Non-specific binding was blocked in 2.5% normal horse serum from the ImmPRESS Horse Anti-Rabbit IgG PLUS Polymer Kit Peroxidase reagent kit (Vector Laboratories, CA, USA, MP-7801) for 20 min. Furthermore, the serum excess was removed from the samples. In the next step, the samples were incubated for 30 min, at 37°C, with a primary Rabbit polyclonal E-cadherin antibody (biorbyt-orb323260, 1 : 200 dilution) and Rabbit polyclonal N-cadherin antibody (biorbyt-orb11100, 1 : 50 dilution). In the next step, the slides were washed in a phosphate-buffered saline (PBS) buffer for 5 minutes. They were then incubated for 30 minutes with the ImmPRESS reagent. Next, the slices were washed for 2 × 5 minutes in PBS buffer. After the buffer was removed from the slides, they were incubated in ImmPACT DAB EqV working solution until the desired staining was achieved. Then, repeated washes in PBS buffer were performed for 2 × 5 minutes. The samples were stained with hematoxylin. Finally, the slides were dehydrated in a series of alcohols, and xylene and cover slides were mounted.
E-cadherin and N-cadherin immunohistochemical reactions were used as a positive control of liver cancer (E-cadherin) and breast cancer (N-cadherin) tissue material.
Negative controls for the immunohistochemical reactions were the aforementioned positive control preparations untreated with the respective primary antibody.
Morphometric analysis
Morphometric analysis was based on the evaluation of microscopic images, using automatic processing and analysis of the immunohistochemical reaction images obtained. The analysis was performed using the commercial cellSens dimension software from Olympus [1].
The morphometric analysis included the number of cells immunopositive for E-cadherin and N-cadherin, as well as the area of immunohistochemical reactions.
Five images of the field of view were taken for each sample at a total magnification of 40×. For this purpose, an Olympus BX 43 light microscope and an XC 30 digital camera were used. Phase analysis of the stained preparation was performed according to the program, consisting of automatic detection of objects based on their color (brown DAB-3.3 chromogen). Based on the threshold values introduced, the software performed automatic classification. The microscope had previously been calibrated using a computing program. The surface area values obtained were expressed in µm2.
Statistical analysis
The collected data were characterized by study and control group (n = 16 for both groups) – the mean and standard deviation were given for each variable, together with the median and quarter deviation if the results of statistical tests of the compared variables were presented. To perform a comparison between a group (test or control) and N-cadherin and E-cadherin, a two-factor analysis was performed in a mixed design with consideration of the interaction effect – with the group serving as the fixed factor and the type of cadherin as the repeated factor. Detailed comparative analyses were performed using the Wilcoxon W test for dependent variables (comparisons between cadherin types) and the Mann-Whitney U test for independent variables (intergroup comparisons). The bivariate analysis additionally provided information about the interaction effect: whether the differences at each level of the two variables were significantly different from each other (e.g., whether the difference between immunohistochemical responses and immunopositive cells for E-cadherin and N-cadherin was detected for only one group or whether the difference was similar in both groups) and how strong this possible effect was. The strength of the effect was characterized by the η2 coefficient (eta square).
The Statistica 13.3 statistical package (StatSoft, Tulsa, USA) was used to evaluate differences in the number of immunopositive cells and the immunohistochemical reaction area.
Results
Morphological and biochemical parameters
Before surgery, morphological and biochemical parameters were assessed in all patients and found to be within the reference ranges.
Histopathological examinations
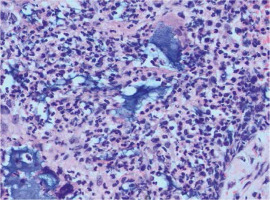
None of the breast tissue samples taken showed neoplastic changes on histopathological examination. In addition, fragments of the mammary gland with fibrosis and lymphocytic infiltration were visible in part of the examined tissue material (Fig. 1). Alkaline, homogeneous Aquafilling filler was found within the adipose, connective, and skeletal muscle tissue. On histological examination, Aquafilling appeared as abundant masses or numerous but small foci.
Morphometric analysis
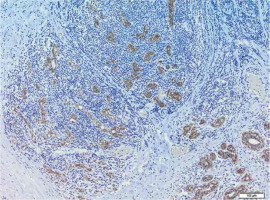
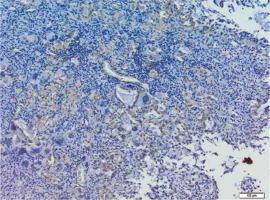
A summary of the average level of particular E-cadherin (Fig. 2) and N-cadherin (Fig. 3) immunopositive cell parameters and the difference between the two cell types by study and control group are presented in Table 1.
Table 1
Characteristics of the mean level of the examined parameters in the study group and control group
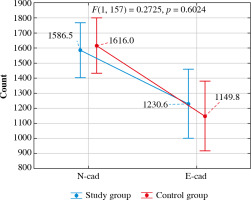
The effect of interaction between the group and cell type for the Cell number variable did not show statistical significance (F(1, 157) = 0.27, p = 0.602) – the number of N-cadherin and E-cadherin immunopositive cells was not significantly different between control and study groups (p = 0.720 for N-cadherin and p = 0.231 for E-cadherin). In contrast, both groups had significantly more N-cadherin than E-cadherin immunopositive cells (p = 0.006 for the study group and p < 0.001 for the control group) (Table 2, Fig. 4).
Table 2
Bivariate analysis of the mean level of the studied parameters (comparison of variables between control and study groups and between immunohistochemical reaction area for N-cadherin and E-cadherin separately for both groups)
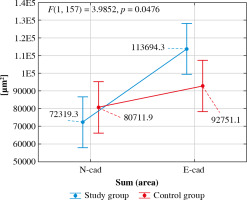
The interaction effect between group and type of immunohistochemical reaction for the Sum (Area) variable was statistically significant (F(1, 157) = 3.99, p = 0.047) – the immunohistochemical reaction area for N-cadherin did not differ between the two groups (p = 0.270), while the immunohistochemical reaction area for E-cadherin was significantly larger in the test group than in the control group (p = 0.021). A significant difference between the immunohistochemical reaction areas was detected in the study group (p < 0.001). Moreover, the reaction area for N-cadherin was significantly smaller than that for E-cadherin. In the control group, no significant difference was detected between the immunohistochemical reaction area for N-cadherin and E-cadherin (p = 0.164). The strength of this interaction effect was η2 = 0.02, which indicated a weak effect of the confirmed interaction (Fig. 5).
Discussion
In the studied tissue material from 16 patients who underwent surgical removal of Aquafilling, no neoplastic lesions were found on histological evaluation. Nonetheless. patients exhibited an aggressive inflammatory profile with long-term complications.
Chronic inflammation can be caused by a variety of biological, chemical, or physical factors. This, in turn, is associated with an increased risk of several types of cancer. Epidemiological and experimental data support the link between chronic inflammation and cancer formation. Examples include inflammatory bowel disease (Crohn’s disease, ulcerative colitis), associated with an increased risk of colon adenocarcinoma, as well as links between chronic pancreatitis and increased incidence of pancreatic cancer [19].
According to information from its producer, BIOTRH s. r. o., Prague, Czech Republic, the Aquafilling filler is a hydrophilic gel consisting of 98% saline and 2% polyamide [20]. When introduced into the human body, it can trigger a response from the immune system. Polyamide is recognized as a harmful exogenous agent by cellular receptors at its injection site. Once it is recognized, inflammatory pathways are activated, and markers are released that cause recruitment of inflammatory process cells. Based on the results of previous studies, the presence of chronic inflammation and very high numbers of immune cells (B lymphocytes, T lymphocytes, and macrophages) at the sites of filler injection has been described. The length of time between the injection of Aquafilling and its removal procedure varied from patient to patient, ranging from 12 to 37 months. Induction of an inflammatory response is most likely an individual trait [1].
In this study, we evaluated the immunohistochemical expression of E-cadherin and N-cadherin in tissue material from a significant number of patients. Histological evaluation revealed no neoplastic lesions. However, given the presence of chronic inflammation, we wanted to investigate whether changes in the number of cells immunopositive for E-cadherin and N-cadherin could be observed, despite the absence of histopathological features characteristic of cancer. Such differences were previously described in EMT. Our immunohistochemical results show a higher number of cells expressing tissue N-cadherin than E-cadherin in the study group. This could support the potential use of both markers in a panel of diagnostic tests to assess early changes occurring at intercellular junctions. In the control group, the number of N-cadherin was also higher than E-cadherin immunopositive cells (p = 0.006 for the test group and p < 0.001 for the control group). During EMT, cell-cell and cell-matrix interactions are remodeled. This results in the separation of epithelial cells from each other and the basal membrane [21]. During tumor progression-associated EMT, E-cadherin expression is lost. Among the many processes occurring during oncogenesis, the impairment of E-cadherin, nuclear β-catenin, and p120 catenin signaling are among the most critical [22]. Reduced expression of E-cadherin leads to carcinogenesis and occurs mainly at the epigenetic level. It has been linked to cellular functions of reduced invasiveness, growth inhibition, apoptosis, cell cycle arrest, and differentiation. Studies on various cancers have shown that these cellular functions are also interdependent [18, 23, 24]. In turn, N-cadherin is an important molecule involved in EMT. It is associated with tumor malignancy [25].
The number of cells showing immunohistochemical expression for E-cadherin was higher in the control (1026 ±482.5) than in the study group (822.5 ±564.75). However, this difference was not statistically significant (p = 0.231). In addition, the immunohistochemical reaction area for E-cadherin was measured, appearing to be larger in the study than in the control group. It is difficult for us to comment on these results due to a lack of reports on this topic in the available literature. However, the fact remains that the number of E-cadherin immunopositive cells was higher in the control group. Perhaps the larger field of immunohistochemical reaction has to do with the course of the inflammatory process, resulting in changes in cell-cell and cell-extracellular matrix interactions. The immunohistochemical reaction for E-cadherin showed a much less continuous and irregular distribution at the cell-cell contact sites, which resulted in a larger response surface.
In turn, the similar immunohistochemical expression of N-cadherin in the study and control groups, as well as the histopathological findings, indicate that EMT does not occur in the breast despite the presence of chronic inflammation. Therefore, surgical removal of Aquafilling should be performed without concomitant subcutaneous mastectomy. As we mentioned in the introduction, breast fat is now recognized as an endocrine organ that secretes several factors [11]. Hence, its complete removal could have further adverse health consequences for these patients.
Conclusions
Immunohistochemical evaluation of tissue expression of N-cadherin and E-cadherin may be useful in assessing early changes occurring at cell-cell junctions. On the other hand, semi-quantitative morphometric analysis allows us to more precisely determine the cell-cell junction differences caused by chronic inflammation induced by Aquafilling.