Introduction
Retinopathy of prematurity (ROP) is one of the most important complications associated with preterm birth. It is a disorder of immature retinal vasculature. It is known that thrombocytes store, deliver and regulate the activity of several key angiogenesis factors, including vascular endothelial growth factor (VEGF) [1]. By that means, as reported by the recent studies, they may limit pathological retinal neovascularization [1, 2].
Retinopathy of prematurity develops in two phases [3] due to the local alternations in VEGF levels and deficiency of insulin-like growth factor 1 (IGF-1) in preterm infants. The birth transfers the baby from the intrauterine environment (PaO2 < 3.5 kPa) to room air (PaO2 = 10.5–12.5 kPa), where increased oxygen concentration leads to physiological retinal hyperoxia and VEGF-downregulation. This results in cessation of retinal vessel growth and vessel retraction in the immature retina – phase I occurs and the retina becomes hypoxic. As the infant grows, endogenous production of IGF-1 rises and activates accumulated VEGF together with retinal hypoxia, leading to proliferative retinopathy (phase II) [3].
Thrombocytopenia is one of the most frequent hematological disorders in neonates. The prevalence of thrombocytopenia is estimated to occur in 1% to 5% of all newborns and 20% to 40% of infants in intensive care units [4, 5]. The results of the study of Ulusoy et al. [4] indicated that thrombocytopenia was detected in 3.8% among all hospitalized neonates and in 12% of pre-terms. Its incidence increases with decreasing gestational age and birth weight.
If platelets limit VEGF accumulation and temper retinal angiogenesis, thrombocytopenia may permit a greater degree of unregulated retinal neovascularization, when IGF-1 levels rise and activate accumulated VEGF (in phase II of ROP).
So far, only a few reports have investigated the role of thrombocytopenia in the development of ROP, none of them has studied its influence on the treatment of the disease.
The aim of the study was to investigate the role of platelet counts, thrombocytopenia, and infections in the pathogenesis of ROP. Furthermore, to determine the critical time of their occurrence and to assess their influence on ROP that requires treatment. We hypothesized that lower levels of platelet count can promote the development and progression of ROP.
Material and methods
Study design and subjects
This is a retrospective study of 163 preterm infants born in the Gynecology and Obstetrics Hospital of Poznan University of Medical Sciences, Poland, between November 29, 2013 and November 28, 2017. The study compared 76 patients who developed ROP requiring treatment (case group: mean gestational age: 25 ±1.72 weeks; mean weight: 830 ±206 g) with 87 patients with ROP that resolved spontaneously (control group: mean gestational age: 28 ±2.07 weeks; mean weight: 1125 ±352 g). The group of patients who required treatment was further divided into the subgroups of patients who after initial treatment recovered from ROP (n = 52), and the group of patients with an aggressive form of the disease who required re-treatment (n = 24).
The study protocol was approved by the Bioethical Review Board of Poznan University of Medical Sciences.
Treatment
Treatment decisions were based on the Early Treatment for ROP (ETROP) study guidelines. Patients with type 1 ROP (zone I any stage ROP with plus disease; zone I, stage 3 ROP without plus disease; or zone II, stage 2 or 3 ROP with plus disease) [6] were treated within 72 h from diagnosis. Laser retinal photocoagulation (n = 47), injection of VEGF inhibitor (n = 5), or both (n = 24) were used as treatment methods. Among the treated patients, in 52 patients, the first line of treatment was sufficient to stop ROP progression, and 24 patients required re-treatment.
Data collection
Thrombocytopenia was defined as a serum platelet count less than 100 × 109/l. The data were collected from electronic medical records. Peripheral blood platelet count measurements from several time intervals were abstracted, including the day of patient’s birth and the most recent platelet count prior to the diagnosis of ROP, qualification to the treatment, and the day of the treatment procedure. The occurrence of thrombocytopenia before 31-week postmenstrual age (PMA), the total number of days with thrombocytopenia (meaning the number of days during hospitalization when platelet count was < than 100 × 109/l), and the number of platelet transfusions were also collected.
The data associated with infectious episodes, included the occurrence of early-onset (intrauterine, < 7 days after birth – congenital pneumonia, congenital sepsis or fetal inflammatory response syndrome (FIRS)) or late-onset infections (> 7 days after birth, any sepsis or ventilator-associated pneumonia).
Statistical analysis
Cases were analyzed in comparison to the control group; further analysis concerned the subgroups of treated patients. Statistical analyses were carried out with Statistica, Statsoft, Inc. 2014, version 12. Mann-Whitney test, χ2 test and Student’s t-test were used to investigate the relationships between variables. The ROC curve analysis and multivariate logistic regression analysis were also performed.
Results
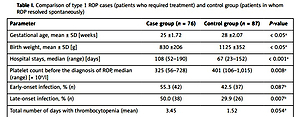
The clinical and demographic data of patients and the comparison among the groups of patients with non-treatment requiring vs. treatment-requiring ROP are shown in Table I. A statistically significant difference between the case and control group was found in the occurrence of thrombocytopenia (p = 0.015) and median platelet count (p = 0.008) before the diagnosis of ROP. The mean postmenstrual age (PMA) for the measurements prior to the diagnosis of ROP was 32.9 weeks; median platelet count in the case and control groups were, respectively, 325 × 109/l (range: 56–728) and 401 × 109/l (range: 106–1,015). The presence of late-onset infection was statistically significant (p = 0.007); no correlation of early-onset infections was found between these groups (p = 0.087).
Table I
Comparison of type 1 ROP cases (patients who required treatment) and control group (patients in whom ROP resolved spontaneously)
| Parameter | Case group (n = 76) | Control group (n = 87) | P-value |
|---|---|---|---|
| Gestational age, mean ± SD [weeks] | 25 ±1.72 | 28 ±2.07 | < 0.05a |
| Birth weight, mean ± SD [g] | 830 ±206 | 1125 ±352 | < 0.05a |
| Hospital stays, median (range) [days] | 108 (52–190) | 67 (23–152) | < 0.001a |
| Platelet count before the diagnosis of ROP, median (range) [× 109/l] | 325 (56–728) | 401 (106–1,015) | 0.008a |
| Early-onset infection, % (n) | 55.3 (42) | 42.5 (37) | 0.087b |
| Late-onset infection, % (n) | 50.0 (38) | 29.9 (26) | 0.007b |
| Total number of days with thrombocytopenia (mean) | 3.45 | 1.52 | 0.054a |
| Platelet transfusions, % (n) | 25.0 (19) | 19.5 (17) | 0.402b |
| No. of platelet transfusions (mean) | 0.697 | 0.368 | 0.198a |
| The occurrence of thrombocytopenia (yes/no), % (n): | |||
| On the day of birth | 5.3 (4) | 4.6 (4) | 0.844b |
| Before 31 PMA | 39.5 (30) | 32.2 (28) | 0.332b |
| Before the diagnosis of ROP | 6.6 (5) | 0.0 (0) | 0.015b |
The analyses including platelets transfusions (p = 0.402), number of platelet transfusions (p = 0.198), the occurrence of thrombocytopenia before 31 PMA (p = 0.332), or on the day of birth (p = 0.844) did not reach statistical significance.
The ROC curve analysis showed that the value of platelets before the diagnosis above 232 × 109/l may promote spontaneous resolution of ROP (the ROC curve: specificity 0.92, sensitivity 0.329; AUC: 0.622, p = 0.063).
Multivariate logistic regression analysis suggested that the risk of ROP development increased with the number of days with thrombocytopenia, with borderline significance (OR = 1.097; 95% CI: 0.99–1.21; p = 0.052) and with decreasing gestational age (OR = 0.523; 95% CI: 0.42–0.65; p < 0.001).
The baseline characteristics of patients once treated and patients who required re-treatment are presented in Table II. Among the cases, statistically significant difference between the patients treated once and the patients that required re-treatment was found in platelet counts before the diagnosis of ROP (mean: 32.7 PMA; median platelet count: 371 × 109/l and 242 × 109/l, respectively; p = 0.017), platelet count before the first intervention (mean: 34.8 PMA; 360 × 109/l and 243 × 109/l, respectively; p = 0.013), and the number of transfusions (mean number of transfusions: 0.48 and 1.17, respectively; p = 0.042).
Table II
Comparison of the subgroups of the patients requiring treatment
| Parameter | Patients treated once (n = 52) | Patients re-treated (n = 24) | P-value |
|---|---|---|---|
| Gestational age, mean ± SD [weeks] | 26.08 ±1.89 | 25.21 ±1.10 | 0.048a |
| Birth weight, mean ± SD [g] | 876 ±222 | 785 ±153 | 0.071c |
| Hospital stays, mean ± SD [days] | 108 ±29.24 | 124 ±30.93 | 0.033c |
| Total number of days with thrombocytopenia, mean ± SD [days] | 2.60 ±5.21 | 5.29 ±8.11 | 0.091a |
| Platelet count before diagnosis of ROP, median (range) [× 109/l] | 371 (86–728) | 242 (56–649) | 0.017a |
| Platelet count before qualification to treatment, median (range) [× 109/l] | 342 (67–728) | 229 (56–678) | 0.099c |
| Platelet count before treatment intervention, median (range) [× 109/l] | 360 (82–656) | 243 (52–557) | 0.013c |
| Number of platelet transfusions, mean ± SD | 0.48 ±1.15 | 1.17 ±1.86 | 0.042a |
| Platelet transfusions (yes/no), % (n) | 19.2 (10) | 37.5 (9) | 0.154b |
| Early-onset infections, % (n) | 53.8 (28) | 58.3 (14) | 0.572b |
| Late-onset infections, % (n) | 50.0 (26) | 50.0 (12) | 0.862b |
| The occurrence of thrombocytopenia (yes/no), % (n): | |||
| On the day of birth | 5.8 (3) | 4.2 (1) | 0.767b |
| Before 31 PMA | 34.6 (18) | 54.2 (13) | 0.077b |
| Before the diagnosis of ROP | 3.8 (2) | 12.5 (3) | 0.157b |
| Before the qualification to the treatment | 1.9 (1) | 12.5 (3) | 0.066b |
| Before the treatment intervention | 1.9 (1) | 4.2 (1) | 0.583b |
There was no significant difference between the patients once treated and the patients that required re-treatment in platelet counts before the qualification for treatment (p = 0.099), in the occurrence of thrombocytopenia on the day of birth (p = 0.767), or before 31 PMA (p = 0.077). The presence of early-onset (p = 0.572) or late-onset (p = 0.862) infections was not significant.
Discussion
The results of our study are in accordance with the previous studies that indicate the association between the development of retinopathy of prematurity and platelet count/thrombocytopenia. While there were no differences in the occurrence of thrombocytopenia right after the birth, its episode before the diagnosis of ROP (mean: 32.9 PMA) seems to be significant for ROP development. Furthermore, higher platelet count before the diagnosis may promote spontaneous resolution of ROP. This study is the first to investigate the role of platelet counts not only in the development of ROP, but we also considered its influence on further treatment. The results of our study suggest that the deficiency of platelets prior to a treatment intervention might be associated with the necessity of re-treatment.
Our findings are in accordance with Jensen et al. [2], who found the relationship between thrombocytopenia closely preceding laser, and the occurrence of type 1 ROP. However, they only analyzed platelet count from one-time interval before the laser therapy (the mean PMA at laser was 37.3 ±2.9 weeks). In our study, platelet counts from several time intervals were analyzed to find the critical time for an episode of thrombocytopenia. Results of our study suggest that deficiency of platelets is significant not only prior to the treatment procedure, but also earlier episodes of thrombocytopenia, before the diagnosis, may play a role in ROP development.
Jensen et al. [7] carried out a longitudinal study on the association between thrombocytopenia and ROP, concerning platelet counts during early (24–28 weeks PMA), middle (29–34 weeks PMA), and late (35–38 weeks PMA), which correspond to the initial hyperoxic, middle hypoxic, and later neovascular periods to occur in ROP pathogenesis. They found a significant association between thrombocytopenia and type 1 ROP in earlier (24–34 weeks PMA), but not later (35–38 weeks PMA) periods of postnatal development. Our results support these findings; the mean PMA for the measurements prior to the diagnosis of ROP was 32.9 weeks, with statistically significant difference in platelet count during this period between the control and the case group.
Lundgren et al. [8] found that both thrombocytopenia and multiple infectious episodes were associated with APROP development. All of the described 9 preterm infants with APROP had thrombocytopenia in the first month of life, the amount of days with thrombocytopenia occurred significantly more frequently, and median platelet counts were lower than in the control group (ROP no worse than stage 2). Similarly, we found that the risk of ROP development increased with the number of days with thrombocytopenia, but with borderline significance. It is possible that the decrease in platelet count may be a response to sepsis, which in turn, according to Lundgren et al. and Guida et al., may be associated with APROP [8, 9].
To date, anemia and blood transfusions were considered the hematologic risk factors for ROP development [10]. Nowadays, low platelet counts, and their increased activity also became of interest as new possible contributors to ROP development. Recently Tao et al. [11] indicated the possible relationship between ROP and mean platelet volume (MPV) as a useful marker of platelet activity. Results of the study suggest that larger platelets may be metabolically and enzymatically more active and by that means, they may play a role in the occurrence of ROP.
In agreement with Vinekar et al. [10] who described spontaneous resolution of aggressive posterior ROP after correcting thrombocytopenia by platelet transfusions, the results of our study confirmed that a higher platelet count may stimulate spontaneous resolution of ROP. Additionally, in our study, the occurrence of thrombocytopenia was significantly more frequent, and the median platelet count before the diagnosis of ROP was lower in the case group. Moreover, the ROC curve analysis suggested the cut-off point between these groups as 232 × 109/l; therefore, platelet count higher than 232 × 109/l may be a good indicator of ROP with a mild course.
Further analysis concerned the subgroups of treated patients. Part of them who required re-treatment had significantly lower platelet counts before the diagnosis of ROP and before the first intervention than patients in whom first line of treatment was sufficient. These results could be interpreted either as positive influence of higher platelets levels on the effectiveness of ROP treatment or lower platelet count on greater severity of ROP. Vinekar et al. [10] also suggested the association between the platelet count less than 100 × 109/l and the development of severe disease (APROP in all 9 of described cases).
Considering the thrombocytopenia as a potential risk factor for ROP development, some authors proposed platelets transfusions as a new treatment strategy of ROP [2, 10].
In this study, the possible role of platelet transfusions was also examined; an indication for platelets transfusion was platelet count less than 50 × 109/l. There was no correlation of platelet transfusions between patients who required treatment and patients with ROP regression, but patients re-treated needed significantly higher mean number of platelet transfusions than patients once treated.
Similar results were presented by Sanack et al. [12] who indicated thrombocytopenia as a predictive variable for ROP in logistic regression analysis (RR = 6.69, 95% CI: 2.83–15.9). Their results showed a significant difference in the occurrence of thrombocytopenia between patients treated and control group (p < 0.001), with no association of thrombocyte transfusions or number of these procedures (p = 0.104, p = 0.073, respectively) between these two groups.
In our study, the occurrence of early- and late-onset infections was compared between the groups. Only late-onset infections were statistically more frequent in patients with ROP that required treatment in comparison to the control group. Moreover, there was no association between occurrence of any infections and requirement of re-treatment. Borroni et al. [13] presented a significant association with the occurrence of ROP and sepsis (bacterial and/or fungal infections, diagnosed by means for a positive culture of blood and/or cerebrospinal fluid), including early and late-onset infections (after the third day of life). In the multivariate analysis, sepsis seemed to be independent factor leading to agressive posterior ROP but was not statistically related to the requirement of laser therapy. According to these results, congenital infections should probably not be suggestive in the course of ROP; however, later infections may be critical in its development.
In conclusion, this study confirmed that the occurence of thrombocytopenia before the diagnosis is associated with ROP development. The deficiency of platelets before the procedure may influence the treatment process. Furthermore, late-onset infections seem to be more significant in ROP development than intrauterine infections. Therefore, preterm infants with the history of thrombocytopenia and infections should be monitored carefully by ophthalmologists.