Introduction
Gastrostomy for nutritional support is nowadays a commonly used procedure that ensures gastric access. It is performed in the patients with swallowing disorders who require a long-term enteral nutrition support. This usually applies to the patients with neurological disorders, mainly those who underwent brain stroke or trauma, sudden cardiac arrest or suffer from a neurodegenerative disease. The second largest group of patients is comprised of the ones with head and neck, oesophageal and gastroesophageal junction cancer. A precondition for performing the gastrostomy is normal small bowel function. Pharyngeal, oesophageal patency and absence of surgical contraindications are additional requirements for endoscopic gastrostomy. The duration of expected enteral nutrition and ethical indications should also be taken into consideration. A correctly performed gastrostomy is a well-accepted method of enteral nutrition that can be easily removed, if necessary.
The first surgical gastrostomy (SG) is believed to be performed by Verneuil, a French surgeon, in 1876; although the idea for this surgery dates back to 1837 and is attributed to Egeberg [1]. The technical aspects of gastrostomy tube placement have been evolving over the years. Nowadays it is most often performed using Kader’s and Witzel’s method. The first percutaneous endoscopic gastrostomy (PEG) conducted in 1979 by Gauderer and described by him 1 year later was undoubtedly a breakthrough in surgical procedures [2]. Currently, PEG has been approved by all clinicians as the method of choice for creating gastrointestinal access in patients with swallowing disorders who require a long-term enteral nutrition. This method allows surgeons to avoid laparotomy with related complications, and to perform this procedure in sedation instead of general anaesthesia. However, it is not a serious perioperative complication-free procedure. Comparative studies demonstrate a higher than originally anticipated percentage of severe complications and mortality after PEG [3–5]. Most of them are retrospective studies comparing SG and PEG [6, 7], while prospective studies are rare [8, 9].
Aim
The goal of this study was to conduct a retrospective analysis of SG and PEG procedures’ efficacy and complications according to Clavien-Dindo classification [10] in high volume groups of patients. The authors also attempted to determine whether one of these methods could be considered safer and more functional for patients.
Material and methods
Study design and data collection
Authors conducted a retrospective analysis of 15-year data on patients with dysphagia, irrespective of cause, who underwent SG or PEG tube placement. All medical records were gathered in our surgical department. Thirty-nine SG and 573 PEG procedures were performed in the examined period of time. SG procedures were performed only in patients with head and neck, oesophageal or gastroesophageal junction cancer with pharyngeal or oesophageal stenosis. The SG and PEG groups of patients were comparable in terms of age: 63.7 ±11.3 years old (43–83 years) vs. 63.4 ±14.1 years old (20–94 years), and body mass index (BMI): 22.5 ±5.3 vs. 21.4 ±4.8. The assessment of nutritional status on NRS 2002 scale prior to the surgery was done in 71.8% of SG patients and in 58.6% of PEG patients. The inability to assess the nutritional status in the entire groups of patients was related to missing data as a main limitation of retrospective analysis. Importantly, contrary to most previously mentioned statistics which were similar in both SG and PEG groups, some differences in nutritional status assessed in NRS 2002 scale were observed: 3.36 ±1.42 vs. 4.46 ±1.42, respectively. The SG and PEG groups of patients differed in sex distribution (15.4% F, 84.6% M vs. 54.5% F, 45.5% M) and indications for gastrostomy, respectively. Detailed data are shown in Table I. Procedure efficacy and duration, type and number of complications according to Clavien-Dindo classification, and mortality rate were analysed. Complications and perioperative mortality were assessed during hospitalization, however not longer than for a month after the surgery. In case of patients discharged from hospital within 1 month, no post-discharge audit was performed. The Ethics Committee approval was not required due to the retrospective nature of the study.
Table I
Patient baseline characteristics and indications for gastrostomy
PEG and SG procedures
Basic blood tests, including CBC, coagulogram, blood glucose test, electrolytes, urea and creatinine, were performed in all patients prior to gastrostomy. Prophylactic antibiotic: cefazoline 1 g or cefuroxime 1.5 g intravenously was administered preoperatively. All PEG procedures were performed by four surgeons. SG procedures, in turn, were performed by several surgeons. Patients stayed at the postoperative unit for a short time after surgery and then were transferred to their initial wards. Enteral feeding through the gastrostomy tube was started after 24 h and 24–48 h in case of PEG and SG, respectively. Bolus infusion procedure or continuous infusion by the pump was used during hospitalization. The type of diet was chosen by the nutritional support team consisting of doctors, nurses and clinical dieticians.
Surgical gastrostomy tube was placed under general anaesthesia by short midline epigastric laparotomy. Gastrostomy was performed using the Kader’s method; the tube was inserted through a skin swab wound and then short stomach wall incision was kept in place with two purse-string sutures. The stoma site was secured with 3–4 single sutures to the peritoneum of the anterior abdominal wall. The gastrostomy drain was sutured to the skin to prevent its dislocation.
Percutaneous endoscopic gastrostomy was performed in all patients using the “pull” method in the operating theatre under intravenous sedation or general anaesthesia. The procedure started with endoscopy of the upper gastrointestinal tract to confirm normal anatomy, exclude local contraindications and determine the area of PEG tube insertion through diaphanoscopy and compression of the abdominal wall. A puncture cannula was inserted into the stomach through 10 mm incision of the skin and abdominal wall under endoscopic control. A thread inserted through the cannula was grasped with biopsy forceps and pulled out together with an endoscope. The oral end of the thread was attached to the end of PEG Flocare tube 18 Ch (Nutricia), which was afterwards pulled into the stomach and out through the abdominal wall. Afterwards it was gently secured with an external stopper and sterile dressing was applied. The endoscope was reintroduced only in limited cases to confirm correct PEG tube placement.
Outcomes
For the outcome measurements we analysed all complications related to gastrostomy according to Clavien-Dindo classification. Complications were divided for ease of statistical analysis into three groups: minor (grade I and II according to Clavien-Dindo classification), serious (grade III and IV according to Clavien-Dindo classification) and deaths (grade V according to Clavien-Dindo classification). Additionally, we analysed the treatment methods of complications.
Statistical analysis
Numerical data are presented as means ± standard deviation unless stated otherwise. Categorical variables were compared using the Pearson’s χ2 test with Yates correction. Continuous variables were analysed using the Mann-Whitney U test. Univariate and multivariate logistic regression has been applied to explain the risk of complications following different treatment types (PEG/SG). The aim of the multivariate analysis was to eliminate the effect of non-random selection of the treatment regime among patients with different nutrition state which might be a threat in the univariate analysis. A p-value less than 0.05 was considered to be statistically significant. Statistical analysis was performed with Statistica software.
Results
The average duration of PEG procedures, from insertion of the gastrofiberoscope to placement of a dressing around the catheter, was 16 ±3.5 min (10–45 min), whereas SG procedure was significantly longer – 90.0 ±38.8 min (35–170 min) (p < 0.01). All elective procedures were successful in SG patients, without intraoperative complications. Two procedures have not been finished successfully in the group of PEG patients. One intraoperative death was noted due to sudden cardiac arrest during induction to general anaesthesia. In the second case, laparotomy was required in the cachexic patient because of too deep skin incision perforating the stomach wall at the beginning of the PEG procedure.
No perioperative mortality was noted in thirty nine SG patients within 30 days after the surgery. However, it should be emphasized that in case of both SG and PEG patients discharged from hospital within 1 month, no post-discharge audit was performed. Grade III and IV complications according to Clavien-Dindo classification (serious) were observed in three SG patients, aged 42–54 years, without serious concomitant diseases but with more advanced cancer disease. The frequency of these complications was statistically significantly higher (7.7% vs. 1.7%, p < 0.01) than in PEG patients. All of these patients developed fibrino-purulent peritonitis caused by leakage in the stoma site; as a reason of septic shock in 1 case and wound dehiscence in the other. All of them were reoperated. The surgical procedures included suturing of the leaking stoma site, lavage and draining the peritoneal cavity. Grade I and II complications (minor) were reported in 10 patients, aged 53–76 years and their frequency was significantly higher (25.6% vs. 0.9%, p < 0.01) than in PEG patients. Most of these patients suffered from concomitant diseases and were at various stages of cancer disease. One patient developed an intestinal fistula as a result of intraoperative release of massive adhesions, which was effectively treated conservatively with parenteral nutrition, suction drainage and skin care around the fistula. Surgical site infection was observed in 6 patients. Gastrostomy tube fell out in 2 cases, and in 1 case it was mechanically damaged. In all 3 patients feeding tubes were successfully replaced without surgery (Table II).
Table II
Complications according to Clavien-Dindo classification within 30 days after PEG and SG tube insertion
| Variable | PEG (n = 573) | SG (n = 39) | P-value |
|---|---|---|---|
| All complications | 25 (4.4%) | 13 (33.3%) | < 0.01 |
| Grade I and II | 5 (0.9%) | 10 (25.6%) | < 0.01 |
| Grade III and IV | 10 (1.7%) | 3 (7.7 %) | < 0.01 |
| Grade V (mortality) | 10 (1.7%) | 0 (0%) | NS |
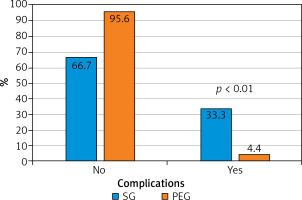
The total number of all complications (grade I–V) in the PEG group of patients was statistically significantly lower (4.4% vs. 33.3%, p < 0.01) than in the SG group of patients (Figure 1). Grade V complications according to Clavien-Dindo classification (deaths) occurred in 10 cases, (including one intraoperative death) out of 573 PEG patients. The total 30-day perioperative mortality rate was 1.74% and did not differ statistically from the one noted in SG patients. Three patients died of peritonitis-related complications caused by PEG leakage. They had to undergo reoperation. One patient died in the course of bleeding from an unresectable oesophageal tumour damaged by PEG tube being dragged along tumour. Five patients died due to exacerbations of underlying or concomitant diseases: another brain stroke, myocardial infarction, amyotrophic lateral sclerosis, pneumonia as a complication of mechanical ventilation and renal failure. All of patients with grade V complications were elderly (aged 60–85 years), mostly with serious concomitant diseases. Ten grade III and IV complications were noted in PEG patients. Their incidence was statistically significantly lower (1.7% vs. 7.7%, p < 0.01) than in SG patients. Two patients developed peritonitis caused by PEG tube leakage, but they were successfully treated surgically. The surgical procedures included suturing the leaking stoma site, lavage and draining the peritoneal cavity. Severe surgical site infections with stoma channel necrosis were observed in 2 other patients, requiring reoperation due to symptoms of peritonitis and sepsis. Acute buried bumper syndrome symptoms, stoma channel necrosis with massive infection of subcutaneous tissue were diagnosed intraoperatively. The iatrogenic perforation of transverse colon was the reason of reoperation in 1 patient with symptoms of peritonitis. Five patients suffered from excessive peristomal leakage effectively treated with PEG tube replacement of a wider low-profile feeding tube or G-tube. Grade I and II complications: surgical site infection usually caused by minor peristomal leakage, developed in 5 patients with PEG and were treated locally (Table II).
A univariate logistic regression was first performed to check the preoperative nutrition state effect (NRS 2002 scale) on the probability of complications. The results indicate no significant effect of the nutrition state on the probability of serious complications (OR = 0.83, 95% CI: 0.51–1.34, p = 0.46). The result remains stable if the missing NRS 2002 values are imputed with sample means for the given treatment (OR = 0.79, 95% CI: 0.49–1.26, p = 0.33) (Table III). At the same time, univariate logistic regression confirms the significant effect of the treatment type on the probability of serious complications – after PEG procedure the risk of complications was notably lower (OR = 0.21, 95% CI: 0.05–0.8, p = 0.02) (Table III). However, the choice of treatment and nutrition state are not independent – univariate logistic regression analysis revealed that worse nutritional status (higher NRS 2002 values) increased the chance that PEG was performed (OR = 2.19, 95% CI: 1.67–2.87, p < 0.001). That is why despite lack of statistical significance of the preoperative nutrition status effect, the NRS 2002 measure was included in the multivariate logistic regression model together with the type of procedure in order to eliminate the preoperative patients’ condition to the greatest possible extent. However, the results given in Table IV indicate that patients who underwent the PEG procedure had a lower risk of serious complications than patients treated with SG (OR = 0.23, 95% CI: 0.05–0.99, p = 0.05) and this result is robust to the preoperative patients’ nutrition status which is not found significant (OR = 0.91, 95% CI: 0.55–1.49, p = 0.71) (Table IV).
Table III
Univariate logistic regression analysis – probability of serious complications according to patients’ nutrition state (*with attrition of the missing data) and type of procedure
| Variable | No pts | OR | 95% CI | P-value |
|---|---|---|---|---|
| NRS 2002 | 364 | 0.83 | 0.51–1.34 | 0.46 |
| NRS 2002* | 612 | 0.79 | 0.49–1.26 | 0.33 |
| PEG vs. SG | 612 | 0.21 | 0.05–0.80 | 0.02 |
Discussion
Our study revealed that the number of patients with all grade I–V complications was statistically significantly lower in the group of PEG patients (4.4%) than in the group of SG patients (33.3%). This difference arises among others from a substantially lower incidence of grade III and IV – serious, potentially fatal complications of PEG (1.7%) vs. SG (7.7%). Statistical analysis of our data confirmed a significant effect of the treatment type on the probability of serious complications (p = 0.02). In the literature, the reported rate of severe, nonlethal complications of PEG and SG procedures is 0–16.7%, 11.1–27.8% respectively [3, 5, 6, 9, 11–15]. Although mean BMI and NRS 2002 nutrition status of PEG patients were insignificantly worse than in SG patients, the results of our statistical analysis indicated no significant effect of the nutrition status on the probability of serious complications. In the investigated group of patients peritonitis caused by gastric leakage around the feeding tube (5 in the PEG group and 3 in the SG group), which required reoperation, was the most common complication among the severe ones reported in the literature [9, 11–13]. It is worth noting that in the SG group they have appeared in relatively young patients with advanced cancer disease, and in the PEG group in older ones mostly in severe general condition. Stoma channel necrosis with massive surgical site infection was the second most common severe PEG complication. This complication associated with a high (50%) reoperation rate was more frequent at the beginning of the PEG procedure learning curve and most probably resulted from excessive pressure between internal and external bumpers. Iatrogenic perforation of the transverse colon and excessive peristomal leakage requiring PEG tube replacement were other grade III–IV complications observed in the study that are reported in the literature [4, 5, 16].
The incidence of grade I and II complications (surgical site infection usually caused by minor peristomal leakage, mechanical damage or dislocation of the feeding tube) was also statistically lower in PEG than in the SG group of patients (0.9% vs. 25.6%). The reported incidence of such complications is up to 40% and 71% in PEG and SG patients, respectively [4, 5, 6, 9, 13–16]. Surgical site infection was more common in SG patients, which is certainly associated with major surgical trauma. Mechanical damage or dislocation of the gastrostomy tube were observed only in SG patients and were caused by balloon rupture or mechanical damage of the tube itself. Minor peristomal leakages were, in turn, observed only in PEG patients. A higher incidence rate may be caused by possible tearing of the stomach wall during introducing a PEG tube and lack of purse-string suture, being a typical procedure during SG.
A 30-day grade V complication (mortality) rate was 1.74% and 0% in PEG and SG patients, respectively and did not differ significantly. However, it should be emphasized that in case of both SG and PEG patients discharged from hospital within 1 month, no post-discharge audit was performed. According to the literature, 30-day postoperative mortality rate ranges from 2.8% to 13.8% in patients after the PEG procedure [5, 6, 15, 17, 18]. In our study 5 PEG patients died of underlying and concomitant chronic diseases. This group comprised many patients who were chronically malnourished due to neurological diseases causing dysphagia. Some of them were insufficiently fed by their family members orally or using a thin nasogastric tube for several weeks before the surgery. Mean BMI and NRS 2002 nutrition status of PEG patients were insignificantly worse than in SG patients and presence of concomitant diseases was more common. Other five deaths should be associated with surgical procedure performed under general anaesthesia or in sedation and its complications: death during induction for surgery, peritonitis and haemorrhage. Grade V complications in the PEG group appeared in elderly patients in severe general condition. These fatal complications of the PEG technique are well known and reported [5, 11, 12]. It is worth noting that PEG patients who required laparotomy in the postoperative period because of peritonitis were at over 30 times higher risk of death (37.5%) than patients who did not require surgery (1.2%). The high risk of death in these patients could also be due to severe general condition. This association between laparotomy and death was highly statistically significant (p < 0.01). No 30-day mortality was observed in SG patients. However, it should be emphasized that although this group of patients was similar in age, but with less common concomitant diseases, and the only indication for SG was head and neck, oesophageal or gastroesophageal junction cancer. There were no patients in severe general condition, which was caused by brain stroke, sudden cardiac arrest or neurodegenerative disease in this group. 30-day postoperative mortality rate reported in the other studies was much higher than in our study and ranged up to 33.3% in SG patients [3, 6, 13]. Authors of these papers believed that poor general condition of patients with cancer disease was the cause of a high mortality rate. However, such a high mortality rate was not confirmed in our study.
The major strengths of our study include the large sample size of patients who have undergone PEG procedure and a comparative type of this study. The limitations of this study are related to all retrospective analyses. The database is restrained to in-hospital events sometimes with missing data and as a result underrepresent the actual incidence of mortality and other complications as they may occur after hospitalization. Nevertheless, with these limitations in mind, we have made several observations which deserve consideration.
Conclusions
Data analysis showed a significantly lower percentage of complications in PEG patients as compared with SG patients, both in terms of grade I–II and III–IV postoperative complications according to Clavien-Dindo classification. Statistical analysis of our data confirmed a significant effect of the treatment type on the probability of serious complications and this result was robust to the preoperative patients’ nutrition status which was found to be insignificant. On the other hand, 30-day perioperative deaths were noted only in PEG patients and half of them were associated directly with the surgical procedures, although they were undoubtedly affected by their severe general condition. A low percentage of postoperative complications, easy procedure which can be performed in sedation make PEG a procedure of choice in patients with swallowing disorders who require enteral nutrition. However, it should be emphasized that these procedures are associated with a risk of severe, including fatal, complications. Therefore, all medical and ethical indications and contraindications to the procedure should be considered in every case.