Introduction
Renal cell carcinoma (RCC) is a heterogeneous disease that includes histological entities characterised by different aetiology, morphology, and outcome. Three histological subtypes: clear cell (ccRCC), papillary (pRCC), and chromophobe (chRCC) account for 85–90% of all RCC cases [1]. The incidence of RCC is constantly increasing in North America and Europe [2] with the exception of Finland and Estonia where it has been stable [3]. The majority of cases (80%) occur after the age of 40 years, and the incidence rate is higher among men compared with women (3 : 2) [4]. A reliable causative agent of RCC is still unknown, but existing evidence points to chromosomal defects contributing to its development [5]. Objective diagnostic biomarkers and effective targeted therapeutics are also lacking [6].
The intensive metabolic activity of tubular epithelial cells, glomerular cells, and activated macrophages make them the main generators of reactive oxygen species (ROS) in human kidneys [7]. At the subcellular level, ROS are produced in the process of oxidative phosphorylation in mitochondria and as a product of membrane-bound protein NADPH oxidases (NOX family) [8]. Under physiological conditions, ROS have a role as signalling molecules, and their physiological level is maintained by an antioxidant (AO) system. The AO system of the kidneys is based on small scavenging molecules like glutathione (GSH) and enzymes, which catalytically modify ROS into less harmful forms. Superoxide dismutase (SOD), catalase (CAT), and the glutathione peroxidase family (GPxs) are the main enzymes involved in AO defence. Glutathione reductase (GR) and glutathione S-transferase (GST) are glutathione-dependent enzymes, which additionally contribute to the AO ability of the kidneys [9]. Oxidative stress is a condition related to kidney cancer [10] and is mediated through a reduction in cellular antioxidant pool [11].
Efforts are still ongoing to find the set of predictive and prognostic markers of survival in patients with RCC [12]. Taking into account the significance of oxidative stress for all three phases of cancer development and the role of the AO system in preventing its process [13], we examined the relation between AO status and overall survival (OS) among RCC patients who underwent radical nephrectomy.
Material and methods
Subjects
We analysed 95 patients with RCC, who underwent radical nephrectomy in several Urology Clinics in Belgrade, Serbia, between 2009 and 2013. The study was performed in compliance with the ethical principles of the Declaration of Helsinki and all applicable national laws and regulations. The clinicopathological features of patients included age, gender, size, grade, stage, and histological classification. For tumour histology, the 2004 WHO classification [1] was used and tumours were graded according to the Fuhrman grading system [14]. OS time was determined from the surgery date until death or the last follow-up appointment. The follow-up period was 5 years (median: 38 months, IQR = 31–42 months). The median age of patients at diagnosis was 62 years (IQR = 58–66.2 years).
Specimens
For the enzyme activity measurement, the tissue was washed in a saline solution (PBS) at pH 7.4 in order to remove blood cells. All samples were homogenised on ice in eight volumes of cold potassium phosphate buffer (0.05 M KH2PO4, 0.0001 M EDTA, pH 7.4) by using Ultra-Turrax Homogeniser (IKA® T10 basic ULTRA-TURRAX®, IKA Werke GmbH & Co.KG, Staufen, Germany) at 25,000 rpm for 15 s in four cycles. Homogenates were left overnight at –70°C to disrupt cell membranes. The thawed homogenates were vortexed for 1 min and then centrifuged (10000 × g, 15 min, 4°C) and the upper layer was collected and kept at –70°C till the assay.
For GSH and total malondialdehyde (MDA) concentration measurement, the tissue was prepared as recommended by the kit producer (BIOXYTECH® GSH-420TM and BIOXYTECH® MDA-586TM respectively, OXIS International Inc., Foster City, CA, USA).
Assays
The protein concentration was measured with PierceTM BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). The method is based on a combination of the biuret reaction and colorimetric detection of cuprous cation (Cu+1) with bicinchoninic acid (BCA). The cupric cation (Cu+2) is reduced by the sample proteins in an alkaline medium to Cu+1. Two molecules of BCA react with one Cu+1 making a purple-coloured product that absorbs at 562 nm. The protein concentration was expressed as mg/ml of the sample.
Total SOD activity was measured using Superoxide Dismutase Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). The assay is based on the reaction between tetrazolium salt and superoxide radicals (O2 .-), which results in formazan dye development. SOD inhibits this reaction by dismutation of O2 .-, generated by xanthine oxidase. The intensity of formazan dye was measured at 450 nm using a microplate reader (Wallac1420 Victor2, PerkinElmer Inc., Waltham, MA, USA). One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of superoxide radical.
Catalase activity measurement was performed by the method of Beutler (1984) [15]. The method is based on the ability of CAT to decompose hydrogen peroxide in an incubation mixture to water and oxygen. The incubation mixture contains 50 μl of a Tris-HCl buffer (1 M Tris-HCl, 5 mM EDTA, pH 8.0), 900 μl of a substrate (10 mM H2O2) and 30 μl of distilled water. The reaction starts after adding 20 μl of the sample. Decomposition of H2O2 was monitored for 3 min at 230 nm and 37°C using a spectrophotometer (UV Line 9400, SI Analytics GmbH, Mainz, Germany). One unit of CAT activity is defined as the amount of enzyme that degrades 1 μmol of H2O2 per minute under assay conditions. The extinction coefficient for H2O2 at 230 nm is 0.071 mM–1cm–1.
Glutathione peroxidase activity was assessed by the BIOXYTECH® GPx-340TM Assay (Oxis International, Inc., Foster City, CA, USA). The assay is based on reduction of organic peroxide, which produces oxidised glutathione (GSSG) immediately reduced to GSH by GR with concomitant oxidation of NADPH to NADP+ and a decrease in absorbance at 340 nm. The absorbance decrease was measured spectrophotometrically, and it is directly proportional to the GPx activity in the sample. The extinction coefficient for NADPH at 340 nm is 6220 M–1cm–1. One unit of GPx activity causes the oxidation of 1 μmol of NADPH per minute under the assay conditions.
Glutathione S-transferase activity was measured using Glutathione S-Transferase Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). The assay utilises the conjugation of CDNB (1-chloro-2,1-dinitrobenzene) and GSH accompanied by an increase in absorbance at 340 nm. The extinction coefficient for CDNB at 340 nm is 0.0096 μM–1cm–1. One unit of GST will conjugate 1 nmol of CDNB with GSH per minute at 25°C.
Glutathione reductase activity was determined using the BIOXYTECH® GR-340TM Assay (OXIS International, Inc., Foster City, CA, USA). The assay is based on catalysed reduction of GSSG to GSH and oxidation of NADPH to NADP+. The oxidation of NADPH results in a decrease of absorbance at 340 nm as a function of time. The molar extinction coefficient for NADPH at 340 nm is 6220 M–1cm–1. One unit of GR activity is defined as the amount of enzyme catalysing the reduction of 1 μmol of GSSG per minute under assay conditions.
Total GSH concentration was determined by the BIOXYTECH® GSH-420TM Assay (OXIS International, Inc., Foster City, CA, USA). The measurement is based on a three-step colourimetric reaction. The reducing agent, TCEP (Tris[2-carboxyethyl] phosphine), reduces all oxidised glutathione present in the sample. In the next step chromogen (4-chloro-1-methyl-7-trifluoromethylquinolinium methylsulfate) is added, reacting with all thiols in the sample and forming thioethers. NaOH raises the pH of the reaction mixture to over 13 and leads to chromophoric thione development as a result of β-elimination specific to the GSH-thioether. The absorbance measured at 420 nm is directly proportional to the GSH concentration.
Total MDA concentration was determined using BIOXYTECH® MDA-586TM Assay (OXIS International, Inc., Foster City, CA, USA). The protocol is based on hydrolysis of the sample in the presence of butylated hydroxytoluene (BHT) at low pH (1-2) at 60°C for 80 min. After hydrolysis, free MDA reacts with the chromogenic reagent (N-methyl-2-phenylindole, NMPI) for 60 min at 45°C. The reaction yields a carbocyanine dye that can be detected at 586 nm.
The enzyme activities were expressed as units (U) or mU per milligram of proteins (U/mg or mU/mg). Concentration of GSH and MDA were expressed as nmol/mg of proteins.
Statistical analysis
Statistical analysis was performed with SPSS 23.0 for Windows (SPSS Inc., Chicago, IL, USA). The OS rate was calculated by Kaplan-Meier method, and groups were compared using Log-rank statistics. The compared groups of AO parameters were made by cut-off points based on median value of enzyme activity or MDA and GSH concentration, as previously reported [16]. Univariate and multivariate Cox proportional hazards regression model were used to analyse the independent factors related to 5-year OS. P-values < 0.05 were considered significant.
Results
Clinical findings
Clinicopathological characteristics of patients are given in Table I. The majority of patients were between ages 61 and 70 years, and both genders were almost equally represented. The median tumour size was 7 cm (IQR = 5–8.2 cm). The majority of tumours were up to 7 cm in diameter (63.1%). Low-grade (I–II) RCC was diagnosed in the majority of patients. Stages 1 and 3 were most frequently recorded, and ccRCC was the most common histological subtype at the time of diagnosis.
Table I
Clinicopathological characteristics of patients with renal cell carcinoma (N = 95)
Survival analysis
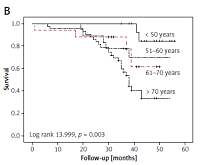
The OS for all patients was 96.8%, 73.7%, and 54.6% after 1, 3, and 5 years, respectively (Figure 1 A). Statistically significant correlations existed between survival and age (log-rank 13.999, p = 0.003), tumour size (log-rank, 22.540, p < 0.001), grade (log-rank 12.080, p = 0.001), and stage (log-rank 26.217, p < 0.001) (Figures 1 B–E).
Figure 1
Survival in 95 patients with renal cell carcinoma. A – overall survival; survival according to: age (B), tumour size (C), Fuhrman grade (D), stage (E), histological subtype (F)
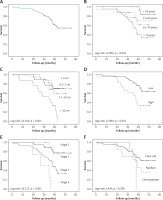
In each age group the 5-year OS rate was as follows: 84.6% among patients younger than 50 years, 70% among patients aged 51–60 years, 33.9% among patients aged between 61–70 years, and 61.8% among those older than 70 years (Figure 1 B). The rates of OS after 1, 3, and 5 years for patients with RCC size ≤ 4 cm were 100%, 77.8%, and 70.7%, respectively; patients with tumour size 4.1–7 cm had survival rates of 97.6%, 87.7%, and 70.4% after 1, 3, and 5 years. After 5 years of follow-up, 60.2% of patients with RCC size 7.1–10 cm were still alive. None of the patients with tumour size > 10 cm were alive after 5 years (Figure 1 C).
After 5 years, survival among subjects with low-grade tumours was 64.3%. In the high-grade tumour group, the survival rate was 96.6, 55.2, and 34.5% after 1, 3, and 5 years, respectively (Figure 1 D). Patients with low-stage tumours (stage 1 and 2) had survival rates of 77.2% and 77.9%, respectively, while 34% of patients with stage 3 were alive after a 5-year follow-up period. None of the patients with stage 4 RCC were alive after 5 years (Figure 1 E). As regards histological subtypes, after 5 years the better survival rate (61.4%) was observed in patients with ccRCC compared to those with the pRCC (36.9%), while chRCC patients showed survival rates of 100%, 64.3%, and 0% after 1, 3, and 5 years, respectively (Figure 1 F). There was no significant association between gender, histological subtype, and survival rate (log-rank 0.002; p = 0.962, log-rank 2.424, p = 0.298, respectively).
The OS analysis based on the AO enzymes (Table II) revealed no statistically significant difference between the median values in survival rates. Also, Kaplan-Meier test showed no significant influence of lipid peroxidation parameter (MDA) and GSH concentration on survival of RCC patients.
Table II
Kaplan-Meier analysis of antioxidant status parameters in patients with renal cell carcinoma
Univariate analysis (Table III) showed that size, Fuhrman grade, and stage were significant prognostic factors for OS. The size (> 10 cm) and high Fuhrman grade (III–IV) remained independent predictors of survival in multivariate analysis. Age, as a confounding variable, was excluded from the analysis.
Table III
Factors affecting overall survival in patients with renal cell carcinoma
Discussion
Oxidative stress plays a crucial role in the pathogenesis of various malignancies, including RCC. ROS can induce the carcinogenesis process and sustain tumour progression by damaging DNA and can directly activate cellular pathways of signal transduction, which are associated with malignant transformation. It was previously demonstrated that changes occurred in the oxidative stress/antioxidant status balance at the cellular level during the tumour growth process [17, 18]. Previous studies have shown significantly reduced expression and activity of AO enzymes in cancerous tissue of the kidneys [19, 20]. Moreover, the enzymatic source of ROS is overactive [21] resulting in increased level of oxidative stress in patients with RCC [22–25]. Based on these findings, permanent oxidative stress is a feature of RCC.
Prognostic significance of AO enzyme activities, MDA, and GSH levels in patients with RCC was not confirmed in this study. However, we observed a prospective influence of GPx, GR, and GSH on survival. Studies indicate the importance of GSH and its metabolising enzyme, γ-glutamyl transferase (GGT), which were found to be elevated in pathological states of oxidative stress and linked to tumour growth and survival. Glutathione is a major cell provider of cysteine, which is especially critical for protein synthesis in rapidly dividing neoplastic cells [26]. The study of Hofbauer et al. [27] showed that pretherapeutic serum GGT was an independent prognostic factor for patients with RCC, with higher levels being associated with worse outcomes. It was shown that contrary to enzyme expression on the luminal surface of secretory and absorptive cells in normal kidney tissue, GGT is expressed over the entire cell surface in RCC. This may subsequently lead to elevated levels of GGT and higher intracellular levels of GSH, which correlate with the tumour burden [27]. However, Ganesamoni et al. [22] observed a lower glutathione concentration in association with higher grade RCC and metastatic disease.
Earlier studies also show different results regarding other AO parameters in RCC. Glutathione peroxidase activity in RCC tissue was reported to be significantly decreased in one study [20] whereas it was unchanged in another [25].
The lipid peroxidation level was found to be higher in RCC than in normal renal tissue, and higher tumour grade was associated with increased lipid peroxidation in tumour tissue [22]. Vieira de Ribeiro et al. [28] found overexpression of SOD-1 in ccRCC extracts, which was explained by the fact that the production of ROS is generally more active in malignant cells. Although higher SOD level and lower CAT in tumour tissue were reported [22], no association of SOD level, histopathological findings, tumour grade, and stage was observed. Some recent findings suggest no correlation between tumour staging and serum levels of SOD, GPx, GST, MDA, and GSH levels [29]. In our study, we did not observe an association between GST level and OS; however, other investigators considered GST to be a highly specific diagnostic marker for primary conventional RCC, in which it is a prognostic marker if the grade is omitted from the multivariate analysis [30].
The findings of this study indicate that the 5-year OS was ~55%, and it was within the range of contemporary data [3]. In univariate analysis, tumour size, pathological stage, and Fuhrman grade were independent predictors of OS. The size and grade remained significantly associated with OS after multivariate analysis. The Kaplan-Meier analysis indicated a possible role of the antioxidant GPx/GR activity and the GSH content for OS in these patients because the obtained significance values were near borderline.
Tumour size is considered as an important determinant of the UICC/AJCC (International Union Against Cancer/American Joint Commission on Cancer) TNM stage, which correlates with renal sinus invasion, metastatic potential, and RCC prognosis [31]. In our patients, the tumours with worse prognosis were larger than 7 cm. The risk of death for patients with tumour size of 7.1–10 cm and > 10 cm was 1.2 and 4.1 times higher, respectively. Studies on large cohorts have shown that in patients with ccRCC, each 1 cm increase in tumour size increased the odds of a high Fuhrman grade (III–IV) compared with a low Fuhrman grade (I–II) tumour by 25% [32]. Although tumour stage was a prognostic marker affecting survival, it was not proven to be an independent parameter in multivariate analysis. The prognostic value of tumour grade observed in our study is in accordance with previous data. Most authors agree that Fuhrman grade and tumour stage are the strongest independent prognostic factors for RCC [33–36].
Some limitations of our study need to be addressed. It is likely that the relatively small number of analysed cases (95 patients) and group heterogeneity may have underestimated the prognostic effect of tumour stage, because the tumour stage is among the most recognised prognostic factors. A larger sample size would also provide a better evaluation of the potential prognostic significance of AO parameters, judging by certain borderline significances observed in this study.
In conclusion, in our cohort of patients, we identified histopathological features of tumours as independent prognostic factors in RCC. No correlation was observed between AO status and OS. New studies are needed to provide better insight into cellular biology of oxidation and AO defence mechanisms, aiming to find prognostic biomarkers for patients with RCC.