Introduction
Regular physical activity is a recognized factor stimulating bone formation. In recent years, osteocytes have been shown to be involved in the metabolism of bone tissue in addition to osteoblasts and osteoclasts. Sclerostin is a marker reflected osteocyte activity [1, 2]. It has been observed that immobilization increases the number of osteocytes secreting sclerostin [3, 4], while mechanical load decreased circulating sclerostin levels [5, 6] and resting in bed increased sclerostin [4]. The circulating levels of sclerostin in professionally trained sports athletes have been evaluated only in a few studies. Higher circulating sclerostin levels were found in amenorrheic and eumenorrheic adolescent female athletes than in non-athletes [7]. Similar results were obtained in a study assessing the impact of weight-bearing and high-impact disciplines on sclerostin levels in elite female athletes [8]. An upward trend in concentrations of circulating sclerostin was also observed during training in professional cyclists. In addition, the concentration of sclerostin correlated with exercise, calcium levels in the urine and muscular activity [9]. It should be noted that no association between sclerostin and vitamin D levels in athletes was found [10]. In some studies, higher vitamin D levels in the practice of different sports [11] were related to greater exposure to UVB radiation during outdoor training [12]. Interestingly, in several studies, no effect of physical activity on the parathyroid hormone (PTH) concentration was found [13–18]. It has been suggested that different physical activity types may contrarily affect the plasma concentration of sclerostin. Lower levels of sclerostin were observed in non-weight-bearing compared to weight-bearing disciplines in males but not in female groups [8]. Moreover, substantial increases of circulating sclerostin levels were observed in untrained healthy young women immediately after physical activity [19]. Single bouts of plyometric exercise did not change biochemical markers of bone metabolism including sclerostin [20].
There is a lack of studies assessing the effects of sex hormones and regular fitness training on circulating sclerostin levels. Therefore, the aim of the study was to analyze the effects of regular fitness training, sex hormones, and selected bone turnover markers on sclerostin levels in young women.
Material and methods
Research type and selection of subjects
Our cross-sectional study involved 78 women, including 39 who regularly engaged in fitness training (for 1 h three times a week for 3 months) and 39 leading a sedentary lifestyle.
First stage of recruitment
Data concerning health status of respondents, the prevalence of any chronic conditions and lifestyle of participants were collected using a questionnaire completed in Sport Gyms in the Silesian Region. Questionnaires were distributed by e-mail with all the information concerning design of studies and anticipated procedures was provided. At the same time the questionnaires were sent to universities in Katowice.
Considering the topic of the present study, the following selection criteria were adopted: regular menstruation, normal thyroid function, stable body mass during the last 3 months, and non-use of hypocaloric dieting during the last 6 months.
Exclusion criteria: Patients using hormonal therapy during the last 3 months, any other pharmacotherapy, acute and chronic diseases (excluding overweight and obesity), smoking, and using more than three alcoholic drinks per week were excluded. At this stage, information involving more than 221 young women was obtained (121 active young women and 98 passive ones).
Second stage of recruitment
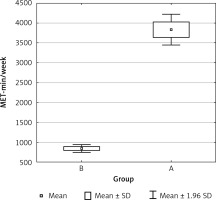
Collected questionnaires were analyzed for the presence of level of physical activity based on the IPAQ Questionnaire short form. PA differences between groups are shown in Figure 1. Only 41 persons were classified to the basic group (A) and 39 were classified in the control group. Two persons quit after recruitment.
Study design
The International Physical Activity Questionnaire (short form – PAQ) was used to assess the effective level of physical activity. It contains 7 questions about activity during the last 7 days related to: professional work, real life, free time and sitting positions. Only activities performed for a minimum of 10 min without interruption are taken into account. The level of physical activity is calculated by multiplying the number of days in a week in which the given physical activity was performed by the time in minutes for a given activity. The obtained result is multiplied by a MET (metabolic equivalent of work) factor appropriate for a given activity. The sum of all the results for individual activities gives a weekly measurement of physical activity, given in a unit of MET min/week. The final result falls into one of three categories defining the level of physical activity: low (< 600 MET min/week), average (600–1500 MET min/week), high (> 1500 MET min/week) [21, 22].
All of the women in the study were tested within 3 and 5 days of their menstrual cycle. Anthropometric measurements (body mass, height, and waist circumference) were performed, and BMI was calculated according to the standard formula. Body composition was assessed by bioimpedance using Bodystat 1500 (Douglas, Isle of Man). 10 ml samples of venous blood were withdrawn in the morning between 8:00 and 9:00 a.m., after an overnight fast (16 h). The blood samples were collected according to recommendations of the manufacturer of the kits. Serum and plasma samples were stored frozen at –70°C.
Biochemical measurements
Plasma glucose and lipids estimate
Plasma glucose and lipids were estimated by colorimetric methods using commercially available test kits (Roche, Switzerland). Serum insulin concentration was determined by the electro-chemiluminescence immunoassay (ECLIA) method using a Cobas E411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany) with a limit of quantification (LOQ) of 0.2 µIU/ml and intra- and inter-assay coefficients of variation of 2.0% and 2.8%, respectively. HOMA-IR was calculated with the standard formula [23].
E2, DHEA, DHEA-S measurements
Estradiol (E2), testosterone, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S) were determined by the ECLIA method using Cobas E411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany) with a lower limit of sensitivity 5.0 pg/ml, 0.025 ng/ml, 0.003 µmol/l and 0.35 nmol/l, respectively; the respective intra- and inter-assay coefficients of variations were 4.6% and 9.9% for E2, 4.7% and 8.4% for testosterone, 2.8% and 4.2% for DHEA and 2.7% and 4.7% for DHEA-S.
Free testosterone and androstenedione measurements
Free testosterone and androstenedione (DRG Instruments GmbH, Marburg, Germany) were determined by ELISA with a lower limit of sensitivity of 0.06 pg/ml and 0.019 ng/ml; and the respective intra- and inter-assay coefficients of variations 10.0% and 10% for free testosterone, 9.1% and 12.1% for androstenedione.
Commercially available ELISA kits were used for measurements of plasma levels of sclerostin (TECOmedical AG, Sissach, Switzerland; the mean intra- and inter-assay coefficients < 4.0% and < 4.8%, respectively), 25-OH-vitamin D (DRG Instruments GmbH for Hybrid XL, Marburg, Germany; inter-assay precision < 14.2%). Osteocalcin, β isomer of C-terminal telopeptide of type I collagen (β-CTx) and iPTH were assessed by ECLIA (Roche Diagnostics GmbH, Mannheim, Germany for Cobas e 411 analyzer) with sensitivity < 3.3%, < 4.2% and < 20% respectively.
Ethics
The experiments reported in the manuscript were performed in accordance with the ethical standards of the Declaration of Helsinki and the participants signed an informed consent form. The study was conducted after obtaining informed consent from each participant. The study protocol was approved by the Ethical Committee of the Medical University of Silesia.
Statistical analysis
Statistical analysis was performed with Statistica 12.0 software (TIBCO Software Inc., Palo Alto, USA). Nominal and ordinal data were expressed as percentages, while interval data were expressed as mean value ± standard deviation in cases of normal distribution or as median with lower and upper quartile in cases of data with skewed or non-normal distribution. Distribution of variables was evaluated by the Shapiro-Wilk test and quantile-quantile (Q-Q) plot, homogeneity of variances was assessed by the Fisher test. For comparison of data between the fitness and control groups, Student’s t-test for independent data (in the case of normal data distribution or after logarithmic normalization, if appropriate, in the case of skewed distribution) or the non-parametric Mann-Whitney U test (in the case of non-normal data distribution) were used. The Pearson correlation coefficient was used as a measure of association between analyzed variables. Multivariable stepwise backward regression analysis was performed for plasma sclerostin levels as an independent variable with potentially explanatory variables: fitness activity, serum levels of estradiol, total testosterone, and DHEA. The Cook-Weisberg test was used to test heteroskedasticity and the Ramsey RESET test was used to test the linearity of regression. The variance inflation factor (VIF) was calculated to check multicollinearity. The goodness of fit of obtained regression models was assessed with the adjusted determination coefficient R2. All tests were two-tailed. The results were considered as statistically significant with a p-value of less than 0.05.
Results
The age and anthropometric parameters of study and control groups were similar. Significantly higher levels of total and LDL cholesterol and estradiol levels were observed in the control compared to the study group, while insulin, androstenedione, PTX and β-CTx levels, and HOMA-IR values were significantly higher in the study group in contrast to the control group. We did not observe differences in HDL cholesterol, triglycerides, glucose, total and free testosterone, DHEA, DHEAS, vitamin D, osteocalcin or sclerostin levels between the study and control groups (Table I).
Table I
Characteristics of study and control groups
| Parameter | Study group, n = 39 | Control group, n = 39 | P-value |
|---|---|---|---|
| Age [years] | 29 ±4 | 29 ±5 | 0.84 |
| Body mass [kg] | 62.0 (56.4–68.0) | 61.3 (56.8–68.9) | 0.91 |
| BMI [kg/m2] | 22.7 (21.3–25.0) | 22.2 (21.1–25.2) | 0.95 |
| Fat percentage (%) | 29.1 (25.6–31.7) | 28.1 (26.6–31.9) | 0.99 |
| Fat mass [kg] | 17.0 (15.0–21.4) | 16.8 (15.6–22.0) | 0.80 |
| Waist circumference [cm] | 76 (72–82) | 76 (72–81) | 0.92 |
| Total cholesterol [mg/dl] | 164.3 ±29.5 | 180.5 ±30.2 | * |
| LDL cholesterol [mg/dl] | 91.5 ±25.3 | 105.0 ±29.0 | * |
| HDL cholesterol [mg/dl] | 57.3 ±12.0 | 60.0 ±10.5 | 0.31 |
| Triglycerides [mg/dl] | 68.4 ±20.4 | 72.5 ±22.0 | 0.40 |
| Glucose [mg/dl] | 86 (79–90) | 83 (80–89) | 0.93 |
| Insulin [µIU/ml] | 7.1 (5.2–10.3) | 6.1 (5.2–7.6) | * |
| HOMA-IR | 1.6 (1.1–2.1) | 1.3 (1.0–1.6) | * |
| Estradiol [pg/ml] | 213 (107–409) | 402 (212–662) | *** |
| Total testosterone [ng/ml] | 0.22 (0.12–0.34) | 0.26 (0.19–0.34) | 0.14 |
| Free testosterone [pg/ml] | 1.2 (0.9–1.8) | 1.1 (0.8–1.7) | 0.35 |
| Androstenedione [ng/ml] | 3.3 (2.8–4.5) | 2.6 (1.8–3.6) | * |
| DHEA [ng/ml] | 6.7 (4.9–8.5) | 6.3 (4.3–9.4) | 0.25 |
| DHEA-S [ug/ml] | 284.1 (228.6–350.6) | 264.9 (218.2–337.5) | 0.46 |
| 25(OH)D3 [mmol/l] | 31.7 ±10.6 | 28.3 ±9.0 | 0.12 |
| iPTH [pg/ml] | 29.2 (16.7–37.9) | 12.4 (6.5–21.7) | *** |
| β-CTx [ng/ml] | 0.53 ±0.18 | 0.43 ±0.14 | ** |
| Osteocalcin [ng/ml] | 20.7 (16.5–24.1) | 19.2 (15.2–22.1) | 0.23 |
| Sclerostin [ng/ml] | 0.40 (0.34–0.51) | 0.41 (0.34–0.45) | 0.37 |
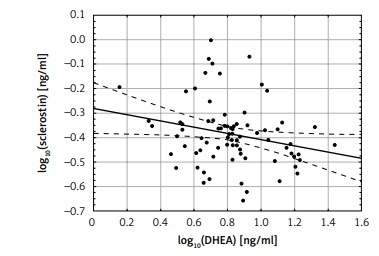
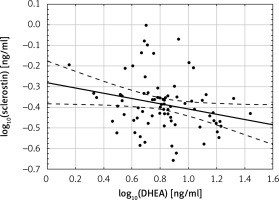
There was no correlation between sclerostin levels and estradiol, total and free testosterone and DHEAS, while there were significant negative correlations between log10 sclerostin and log10 DHEA levels (r = –0.24; p < 0.05) (Figure 2).
A multivariate stepwise backward linear regression model for sclerostin as an independent variable, with the explanatory variables fitness physical activity and estradiol, testosterone and DHEA levels did not reveal effects on changes of sclerostin levels. The model with the explanatory variables vitamin D, iPTH, β-CTx and osteocalcin also did not show an effect on changes of sclerostin levels.
Discussion
Our study is the first assessing the effect of regular fitness training in young, healthy women on circulating sclerostin levels. We did not observe differences in sclerostin levels between healthy young women regularly practicing fitness training and those leading a sedentary lifestyle. This is in accordance with a study performed among female elite athletes in non-weight-bearing and weight-bearing disciplines. In the same study training in weight-bearing, but not non-weight-bearing disciplines increased circulating sclerostin levels in elite male athletes [9]. It should be noted that the level of physical activity in our study group was lower and had an aerobic, recreational character. Thus, the lack of impact of regular fitness training in our study can be explained by both female sex and the type of physical exercise. We cannot exclude that sclerostin levels increased only temporarily immediately after physical exercise but not permanently. This hypothesis is confirmed by a study that showed a substantial increase of circulating sclerostin levels in untrained healthy young women immediately after 45 min of a low-speed, treadmill running test [20]. On the other hand, a single bout of plyometric exercise did not change sclerostin levels [24]. Both of these types of exercise are aerobic; thus the factors increasing post-exercise sclerostin levels in women seem to be more complex than only the type of exercise. Our study has shown that sclerostin levels also do not depend on sex hormones levels and/or levels of 25-OH-D, iPTH, β-CTx, or osteocalcin. These results are contradictory to those obtained by Amrein et al. [25] which demonstrated that sclerostin levels negatively correlated with osteocalcin levels in healthy subjects. In accordance with results described in previously published studies [7, 12] we did not observe an association between sclerostin and 25-OH-D levels.
Similarly to previously published studies [19, 26] we observed higher β-CTx levels in our study compared to the control group. In women undergoing 30 min of exercise by treadmill walking, with an intensity of 50% of heart rate reserve, at a frequency of 3 times a week over a 3-month period, decrease of β-CTx levels after 1 and 3 months was observed [27]. These differences are difficult to explain and further studies are necessary to clarify the effect of different types of training on circulating β-CTx levels. However, previous and our results suggested that different types of exercise are the main factors influencing β-CTx levels. An interesting and difficult to explain finding is iPTH levels over 2 times higher observed in women practicing fitness training compared to women with a sedentary life style. These results are contrary to those obtained in male professional football players, who have lower PTH levels than non-athletes [12], and in several studies that show no effect of physical activity on the PTH concentration [14–19]. The higher PTH levels in our study group cannot be explained by vitamin D deficiencies, but may be a result of lower calcium intake. In accordance with previous observations [12, 28–31] and contrary to others [32], we found no association between circulating sclerostin and iPTH levels. In addition, in our study there was no association between sclerostin and estradiol and testosterone levels. This is in accordance with another study that showed no changes of sclerostin levels during the menstrual cycle [33]. In our study, a negative correlation between sclerostin and DHEA was found. It seems that in accordance with a previously formulated hypothesis [10], there is a relationship between muscle activity, in the absence of load, and bone resorption [34]. Bicer et al. suggested that the cell damage caused by acute exercise in the bone tissue could be prevented by melatonin supplementation [35]. According to Willems et al., multiple dietary components potentially affect osteocyte function and may have an effect on bone health when combined with a regime of PA [36]. In summary, regular fitness training does not change sclerostin levels, and in young women, sclerostin levels are also not affected by circulating estradiol and testosterone levels or 25-OH-D, β-CTx, iPTH and osteocalcin levels. Further studies are necessary to explain factors including type of exercise influencing circulating sclerostin levels in young women.
The natural need for physical activity at a young age should be used to promote health and controlled sport disciplines, especially up-to-date like fitness. On the one hand, of particular importance is the standard formation of active young women investigation. The ability to control and regulate menstrual dysfunction has a fundamental role in the efficient and safe execution of many activities and special motor tasks. Better physique is perceived by others as a sign of good health and self-control. However, it is not uncommon for unrealistic, unattainable image goals to cause the development of eating disorders or other maladaptive perceptions of self [37]. These factors could be a cause of young women’s menstrual dysfunction [38]. On the other hand, cultivated physical activity is significant, especially because nowadays young people are leading a sedentary lifestyle [39].
The main limitations of the study were the small study groups and assessment of sclerostin and bone turnover markers after only 3 months of fitness training. Furthermore, we did not assess calcium and phosphorus levels or calcium, phosphorus, and vitamin D intake. Moreover, bone density was not analyzed.
In conclusion, our results show that regular fitness training, sex hormones, vitamin D, iPTH, β-CTx, and osteocalcin did not influence circulating sclerostin levels in young women.