Introduction
Malignant tumor is one of the main diseases that severely threaten human health and impede social development. According to the latest cancer statistics, about 1.9 million new cancer cases and over 600,000 cancer deaths are projected to occur in the United States in 2022. In recent years, the mortality of cancer has witnessed a consistent decrease all over the world due to the advances in cancer diagnosis and treatment [1, 2]. However, conventional chemoradiotherapy tends to induce dramatic toxic and side effects. Therefore, the high specificity, low side effects, and enduring curative effect immunotherapy based on regulating the immune system have been broadly researched recently. However, due to the complex immune evasion mechanism of tumor cells, such as low expression of tumor associated antigen and the recruitment of immuno- suppressive cells including regulatory T cells (Treg), tumor associated macrophages (TAMs) and myeloid- derived suppressive cells (MDSCs) [3], many patients show lower or no response to cancer immunotherapy. Only a tiny proportion of the patients suffering from malignant tumor can achieve benefit from immunotherapy [4]. Therefore, conducting research on novel targets and new therapies of malignant tumors has never been so urgent.
Triggering receptor expressed on myeloid cell-2 (TREM2) is a transmembrane receptor of the immunoglobulin (Ig) superfamily which is mainly distributed on the surface of myeloid-derived dendritic cells (DCs) and macro- phages. Generally, TREM2 mediates signal transduction via adaptor proteins DNAX-activation protein 12 (DAP12, also known as tyrosine kinase binding protein) and plays an important role in a number of physiological functions such as promotion of macrophage phagocytosis, maturation of DCs and development of osteoclasts. Additionally, TREM2 plays a role in many pathological processes including diabetes, Alzheimer’s disease and cancer [5]. The functional and pathological significance of TREM2 is most clearly studied in Alzheimer’s disease, where variants of TREM2 are associated with increased risk of Alzheimer’s disease [6]. The main pathological features of Alzheimer’s disease are extracellular deposition of amyloid β (Aβ), neurofibrillary tangles and brain neuroinflammation, which is mainly caused by the immune response of settled microglia cells to pathological products. By recognizing Aβ, lipoprotein, apolipoprotein, and some damage-related molecular patterns, TREM2 plays a crucial role in microglia phagocytosis, proliferation, survival, autophagy and energy metabolism [7]. Once TREM2 is mutated, microglia show reduced proliferation and phagocytosis, along with a significant decrease in cell survival, preventing microglia from performing their normal protective function both in number and effect, thus accelerating the progression of the disease [8]. In addition to microglia, TREM2 also performs a certain function in tissue-resident macrophages, such as those in hair follicle stem cell nests, adipose tissue, and bones (osteoclasts) [9].
However, it remains largely unknown whether TREM2 is important in myeloid immunosuppression in the tumor microenvironment (TME). Many seminal studies clearly show that TREM2 is exclusively expressed by myeloid cells rather than non-myeloid cells in TME [10, 11]. Myeloid cells are an important part of innate immunity and play an important role in tissue homeostasis. They can also activate adaptive immunity such as T-cell immune responses. During tumor genesis and development, the body recruits a variety of myeloid cells into the TME by secreting multiple chemokines (such as CCL2 and CCL5). Myeloid cells may directly act on tumor cells or remodel the tumor microenvironment to promote or inhibit tumor progression after infiltrating the tumor. Recently, a paper published in Cell Press demonstrated that TREM2 expressing myeloid subsets were key regulators of antitumor immunity [12]. Meanwhile, TREM2+ myeloid cells express higher levels of immunosuppressive genes such as interleukin (IL)-10, transforming growth factor β (TGF-β) and arginase 1 (Arg1) than TREM2– subsets which may remodel the tumor myeloid landscape. Absence of TREM2 in tumor or anti-TREM2 therapy could restrain the growth of a solid tumor. These findings provide an important theoretical basis for the emergence of TREM2 as a new target for tumor immunotherapy.
Structure of TREM2 and related signaling pathway
The Trem2 gene, encoding a transmembrane protein with a length of 230 amino acids, is located at 6p21.1 on human chromosome 6. TREM2 consists of three domains including an extracellular domain, a transmembrane domain and a short cytoplasmic tail (Fig. 1). The extracellular domain contains a V-type immunoglobulin domain, which can recognize ligands such as pathogen-associated molecular patterns (PAMP), damage-associated molecular patterns (DAMP), cell debris, lipids and apolipoproteins (Table 1). The extracellular domain of TREM2 is cleaved by a disintegrin and metalloproteinase (ADAM) 10 and ADAM17 and released into the extracellular matrix in the form of soluble TREM2 (sTREM2), thereby regulating the expression of TREM2 on the cell surface and may serve as a therapeutic approach. The specific function of this soluble form will be explained in the following paragraphs. The second domain of TREM2 is the transmembrane domain which binds with DAP12. This transmembrane domain can phosphorylate the immunoreceptor tyrosine-based activation motif (ITAM) in DAP12 by associating its lysine residues with the aspartic acid residues in DAP12 and stimulate a series of downstream signaling cascades. The third part of TREM2 is a short cytoplasmic tail without any motifs of signal transduction and transport. Once TREM2 is cleaved by α-secretase such as ADAM10 or ADAM17, the remaining transmembrane portion will further be cleaved by γ-secretase and release this domain. This additional shearing process may further block the signaling of TREM2. However, the specific function of the released domain is largely unknown and needs further research.
Fig. 1
Structure of TREM2 and related signaling pathway. After binding to the ligand, the extracellular domain of TREM2 transmits a relevant signal to its associated DAP12 via the lysine residue in its transmembrane domain followed by the phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) in DAP12. This process leads to the activation of spleen tyrosine kinase (SYK) thus activating a cascade of downstream signaling pathways, including phospholipases C (PLC) activation induced calcium ion mobilization and activated Ras gene mediated extracellular regulated protein kinase (ERK) activation
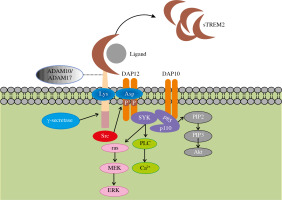
Table 1
Reported TREM2 ligands
| Type of ligands | Ligand’s name | References |
|---|---|---|
| Bacterial components | Lipopolysaccharide (LPS) Dextran sulfate | [18-20] |
| Mammalian | Heat shock protein (HSP60) TREML1 Apolipoprotein E (APOE) Lipoparticles Aβ | [21-24] |
| Anionic molecules | DNA Phospholipids (phosphatidylserine, cardiolipin, etc.) | [25-27] |
Binding of TREM2 to DAP12 mediates phagocytosis and elimination of cell debris as well as proliferation and survival of microglia. Moreover, DAP12 can be activated by Src kinase instead of SYK. In this process, junction proteins Grb2 and Sos are recruited while the ras/MEK/ERK pathway is inhibited. As a result, the secretion of pro-inflammatory cytokine is suppressed [13]. This dual regulation of ERK signaling illustrates the complexity and importance of TREM2/DAP12 pathway homeostasis. With the assistance of DAP10, SYK activates two subunits of PI3K, P85 and P110, mediating the activation of various downstream signaling pathways. In addition, as essential negative regulation of inflammation, TREM2 is involved in both innate and adaptive immunity [14]. In the liver, TREM2 in Kupffer cells induces inflammatory regression by downregulating the phosphorylation of mitogen-activated protein kinase (MAPK), while this anti-inflammatory response is achieved by inhibiting NF-κB signaling in DCs [15, 16]. In other cells, TREM2 also mediates anti- inflammatory responses through the signaling pathways mentioned above [17].
sTREM2
As mentioned above, sTREM2 is the soluble form of TREM2. sTREM2 could act as a biomarker for degenerative neuropathy. The concentration of sTREM2 in cerebrospinal fluid has been proved to correlate with the degree of neurodegeneration and pathological fibrosis of tau protein [28]. Furthermore, sTREM2 is proven to have a unique biological function. It has been shown to promote myeloid cell survival in the case of colony stimulating factor 1 (CSF1) deficiency [29]. This kind of soluble product of cleaved TREM2 may increase the neuroprotective effects of microglia by preventing their apoptosis or promoting their survival. These two opposite effects are mediated through the Akt/GSK3β/β-catenin signaling pathway and the PI3K-Akt signaling pathway, respectively [30]. Moreover, contrary to cellular TREM2, sTREM2 is suggested to promote NF-κB signaling activation and inflammatory cytokine production [31].
Functions of TREM2
TREM2 is widely expressed on myeloid cells, including microglia, osteoclasts, macrophages and DCs. Single-cell RNA transcriptome sequencing showed that TREM2 is also specifically expressed in some tissue-specific macrophages, such as macrophages in adipose tissue, adrenal glands, and placenta [32]. Abundant evidence has suggested that TREM2 plays an important physiological role in the development and function of brain and bone as well as adipose tissues by regulating TREM2-expressed myeloid cells, such as microglia, macrophages and osteoclasts.
Firstly, TREM2 plays an anti-inflammatory role in many cells. In the liver, TREM2 is mainly expressed on the surface of non-parenchymal cells such as hepatic sinus endothelial cells, Kupffer cells and hepatic stellate cells, as well as some cells recruited to injury sites of the liver, such as monocyte-derived macrophages and neutrophils. TREM2 suppresses chronic inflammation in the liver by inhibiting toll-like receptor 4 (TLR4)-mediated inflammation, preventing further progression of chronic inflammation to fatty liver, hepatic fibrosis and hepatocellular carcinoma [33, 34]. The secretion of anti-inflammatory cytokines of macrophages is found to be induced by TREM2 via triggering cascade signaling, which contributes to the phagocytosis of macrophages [35]. A similar function also exists in DCs; the TREM2/DAP12 signaling pathway upregulates the expression of chemokine receptor 7 (CCR7), a significant receptor that contributes to the chemotaxis of cells, by regulating expression of PI3K and ERK [36]. Although the anti-inflammatory effects of TREM2 have been well established, it could theoretically lead to immune activation due to its capacity of binding to components of bacteria. Previous research revealed that TREM2 contributed to the progression of mucosal inflammation in colitis, where TREM2–/– DCs show lower expression of inflammatory factors [20]. Therefore, the bacteria- activated TREM2 signaling pathway needs to be further studied.
Secondly, TREM2 may contribute to the survival and function of microglial cells and osteoclasts. Research based on Nasu-Hakola disease (NHD) has proved the association of TREM2 with microglia and osteoclasts in which TREM2 might play an important role in maintaining normal brain function and bone development [37].
Finally, TREM2 plays a vital role in metabolism of many cells. In adipose tissue, TREM2 maintains the normal function of lipid-associated macrophages, avoiding the metabolic abnormalities associated with a high-fat diet [38]. TREM2 in tissue-associated macrophages acts as a pattern-recognition receptor for common signals indicating loss of metabolic homeostasis, thereby preventing lipid peroxidation (LPO) and promoting cell survival and overall anti-inflammation. Moreover, deficiency of TREM2 affects the transport and metabolism of cholesterol in microglia and caused less phagocytosis of Aβ [39]. A recent study found that loss of serum amyloid A3 (Saa3) in male mice increased the expression of TREM2 in macrophages, thus increasing cholesterol efflux and thereby slowing the progression of atherosclerosis. Interestingly, this process was sex-dimorphic: female mice lacking Saa3 showed opposite regulation of TREM2 and no alleviated progression of atherosclerosis [40].
TREM2 in cancer
Although previous studies have shown that TREM2 had a protective role in central nervous system development, two recent studies indicated that TREM2 was more likely to play an immunosuppressive role in TME, negatively regulating the antitumor immune response and assisting immune escape of tumor cells [12, 41]. As mentioned above, TREM2 can recognize a variety of ligands, including both physical and pathological products (such as ApoE and LDL, which are physiologically present in the body, and Aβ, which is deposited in AD brains). However, whether TREM2 can bind a specific pathological product of cancer needs further research. More relative research needs to be conducted. Implementation of blocking a specific ligand of TREM2 in cancer may be a great improvement of cancer immunotherapy.
TREM2 regulates the expression of anti-inflamma- tory genes and inhibits pro-inflammatory responses, which may be associated with the development of tumors since persistent chronic inflammation could progress to cancer. In the 19th century, Virchow proposed a correlation between inflammation and tumors, and suggested that chronic stimulation of inflammation might be a precipitator of tumor formation. While acute inflammation mediates the host defense against infections, chronic inflammation can predispose the host to various chronic illnesses, including cancer [42]. The expression of TREM2 could inhibit the process of chronic inflammation turning into cancer, thereby preventing the development of tumors. TREM2 is highly expressed in esophageal carcinoma cells and directly maintained their survival [43]. Downregulation of TREM2 in renal cell carcinoma blocks G1 phase and promotes apoptosis of tumor cells, thus inhibiting tumor development [44]. The elevation of TREM2 in gastric cancer is closely related to the aggressiveness, pathologic grade, and histologic type of the tumor, while TREM2 knockout is characterized by inhibiting proliferation, migration, and invasion of tumor cells [45]. TREM2–/– mice have impaired tumor growth compared with wild-type mice, which might be associated with increased infiltration of cytotoxic T and NK cells and depletion of the exhausted phenotype of CD8+ T cells. These findings highlight the importance of TREM2 as a tumor promoter in tumors, but it is worth noting that other studies have reported the role of TREM2 as a tumor suppressor in cancer. TREM2 is reported to inhibit the proliferation and metastasis of colon cancer cells by downregulating cyclin D1 and the Wnt/β-catenin pathway [46]. Other research showed that TREM2 restrained epithelial-mesenchymal transformation (EMT), thus suppressing metastasis of hepatocellular carcinoma cells [47]. Recent research carried out by Biodonostia Health Research Institute also emphasized the protective role of TREM2 in hepatocarcinogenesis [48].
Apart from its immune role in cancer, several studies have focused on its role of cancer diagnosis. Shi et al. found a specific radioligand of TREM2 which could be used to distinguish a tumor from inflammation via positron emission tomography/computed tomography (PET/CT) [49].
TREM2 and tumor infiltrated myeloid cells
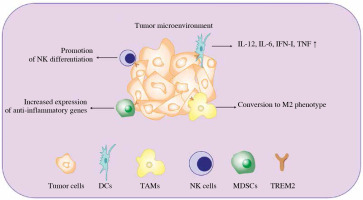
Apart from directly promoting cancer cell survival, TREM2 is also expressed on some tumor infiltrated myeloid cells and regulates tumor development (Fig. 2).
Fig. 2
Diverse function of TREM2 in tumor infiltrated myeloid cells (TIMs). Once the tumor occurs, multiple myeloid derived immune cells will be recruited to the TME in order to restrain tumor development. The expression of TREM2 in TIMs can regulate their function via different approaches, such as regulating their differentiation, phenotypic transition and cytokine secretion. IFN – interferon, TNF – tumor necrosis factor, DCs – dendritic cells, TAMs – tumor associated macrophage, NK cells – natural killer cells, MDSCs – myeloid derived suppressor cells, TREM2 – triggering receptor expressed on myeloid cell 2
TREM2 and dendritic cells
Dendritic cells from tumor-bearing hosts often exhibit phenotypic changes and functional impairment, thereby assisting tumor cells in evading immune surveillance. Ito et al. found that TREM2 deficient DCs secreted higher levels of IL-12, IFN-I, TNF and IL-6 than wild-type DCs after being stimulated by TLR ligands. In addition, compared to the DCs from wild-type mice, DCs derived from bone marrow of TREM2 knockout mice were more efficient in inducing T cell proliferation after TLR ligand stimulation [50]. These findings suggest that TREM2 can regulate TLR-mediated immune responses in DCs negatively. In addition, a recent study confirmed that the expression of TREM2 in nephritis DCs could contribute to nitric oxide (NO) production which negatively regulated the differentiation of Th17 and alleviated the progression of chronic kidney disease [51]. Although TREM2 was originally considered an important anti-inflammatory molecule, several studies have demonstrated that it has both anti-inflammatory and pro-inflammatory effects. Research published by Jiang et al. illustrated that DCs could promote bone destruction in a mouse model of cholesteatoma through increasing expression of TREM2 [52]. The expression of TREM2 on DCs in lymph nodes and lungs is also significantly increased during allergic asthmatic inflammation [53].
TREM2 and natural killer cells
Natural killer (NK) cells are a population of important anti-tumor immune cells, which can kill tumor cells by secreting perforin and granulation enzyme directly. Compared with wild-type mice, hematopoietic stem cells from TREM2 overexpressed mice showed a higher tendency to differentiate into NK cells and showed increasing expression of NK cell-activated receptors and NK cell-related genes. The transfer of bone marrow cells from TREM2 overexpressed mice to tumor-bearing mice significantly restrains tumor progression [54]. These observations suggest that TREM2 enhances the differentiation and function of NK cells, and through this mechanism, TREM2 may fulfil an antitumor role.
TREM2 and macrophages
Macrophages play an important role in immune defense and have a wide range of biological functions, which can trigger both innate immunity and adaptive immunity. The main biological functions include physiological functions such as phagocytosis, antigen processing and presentation, and clearance of apoptotic cells. Macrophages also participate in many pathological processes, including promotion of the inflammatory response, killing of tumor and virus-infected cells and wound healing. In general, macrophages can be divided into M1 type and M2 type according to their functions. M1 macrophages are characterized by high expression of major histocompatibility complex class II (MHC II) and costimulatory molecules such as CD80 and CD86. They are important immune effector cells induced by LPS and IFN-γ stimulation. M2 macrophages on the other hand are characterized by high levels of expression of CD163 and CD206, induced by IL-4 and/or IL-13 stimulation, and illustrated significant impairment of antigen presentation ability and lack of inflammatory properties [55]. TAMs are believed to be one of the most important populations infiltrated in TME, related to poor prognosis and low response to immunotherapy [56]. TAMs accelerate tumor angiogenesis and self-repair and promote tumor pathogenesis via secretion of epidermal growth factor (EGF) and immunosuppressive cytokines (IL-10, arginase-1, TGF-β, etc.) [57].
TREM2 inhibits TLR ligand-mediated secretion of TNF-α and IL-6 in macrophages, and overexpression of TREM2 in myeloid cells attenuates secretion of TNF and inducible NO, promoting IL-10 production. A recent study on sepsis found that TREM2 regulated the immune function of bone-marrow derived macrophages and enhan- ced the defense against bacterial infections by altering reactive oxygen species (ROS) production [35].
A recent paper published in Cell provided evidence that TREM2 is a marker of TAMs and monocytes. The study found that the expression of TREM2 was significantly upregulated in TAMs of tumor-bearing mice and positively associated with tumor progression. It also demonstrated that TREM2 was associated with polarization of TAMs; the researchers detected a decrease in the proportion of immunosuppressive macrophages labeled by MRC1 and CX3CR1 and an increase in the proportion of iNOS+ inflammatory macrophages after TREM2 blockage [12]. In another study, non-small cell lung cancer (NSCLC) patients with high infiltration of TREM2+ TAMs showed advanced tumor progression and poor response to programmed death-1 (PD-1) based therapy [58].
TAM receptors such as Tyro3, Axl and Mer promote M2-like macrophage formation in TME [59]. It is possible that TREM2 can interact with these receptors to promote TAM maturation by recognizing lipids, apolipoproteins, and apoptotic fragments released in TME.
TREM2 and myeloid-derived suppressive cells
Myeloid-derived suppressive cells (MDSCs), a group of immature myeloid cells, are recruited into the tumor microenvironment during tumorigenesis and inhibit the response of anti-tumor T cells. In the tumor-bearing host, reduced recruitment and increased differentiation of immunosuppressive MDSCs significantly inhibit the tumor growth and metastasis [60]. TREM2 can regulate the expression of anti-inflammatory genes (such as Lgals1, Lgasl3, Il1rn, and Grn) and antagonize pro-inflammatory responses associated with tumorigenesis and progression [38]. In TME, TREM2+ myeloid populations show stronger immunosuppressive function, suggesting that TREM2 is involved in the acquisition of immunosuppression in MDSCs.
Recent studies have shown that tumor-associated granulocytic MDSCs (G-MDSCs) and monocytic MDSCs (M-MDSCs) fueled themselves by enhancing fatty acid oxidation (FAO) instead of glycolysis, and the immunosuppressive function of MDSCs was significantly suppressed after treatment of FAO inhibitors [61]. Meanwhile, fatty acid transporter (FATP) promoted the immunosuppressive activity of G-MDSCs by increasing the uptake of arachidonic acid and the synthesis of prostaglandin E2 [62]. As a ligand of multiple lipoproteins and apolipoproteins, TREM2 may regulate the function of MDSCs by reprogramming lipid metabolism.
TREM2 and cancer immunotherapy
Increasing evidence has shown that elevated expression of TREM2 is associated with tumor progression and poor prognosis in gastric, glioma and hepatoma carcinoma patients [63-65]. Molgora et al. found that TREM2 could be a potential target for tumor therapy by eliminating the number of immunosuppressive TAMs [41]. Xiong et al. found that a TAM population with high expression of TREM2 in melanoma might be more resistant to immune checkpoint therapy by analyzing melanoma patient data in the single-cell RNA sequencing (scRNA-seq) dataset [66]. In consideration of its striking regulation of the antitumor immune response, there is a current clinical trial (Clinical Trials No.: NCT04691375) investigating the safety, tolerability and efficiency of a drug named PY314 which targets TREM2 in cancer immunotherapy. Moreover, the pharmacokinetics and pharmacodynamics of PY314 in patients with advanced solid tumors, given alone and in combination with an anti-PD-1 antibody (such as pembrolizumab), are also being investigated. Current data have shown that PY314 could specifically deplete the TAM population with high expression of TREM2, promoting immune responses within the tumor microenvironment by augmenting infiltration of T lymphocyte [67].
Discussion
Accumulating evidence suggests an important role of TREM2 in promoting an immunosuppressive tumor microenvironment and accelerating tumor development. TREM2 is expected to become a new target for tumor immunotherapy [68-70]. Even though little clinical research on targeting TREM2 in cancer is being conducted now, the potential for clinical translation of anti-TREM2 therapy remains vast. Preclinical studies of PY314 have shown antitumor effects of anti-TREM2 therapy in breast cancer. This may be because PY314 reduces the infiltration of pro-tumorigenic M2 phenotype macrophages, while increasing the infiltration of CD8+ T cells, NK cells and M1 phenotype macrophages [71]. Numerous interventions are possible to block TREM2 and its related signaling pathway, such as blocking the extracellular domain with a specific monoclonal antibody (mAb), deleting TREM2 ligands that conditionally existed in the tumor or directly inhibiting TREM2 downstream molecules involved in a related signaling pathway. TREM2 mAb is proved to restrain tumor progression when treated in combination with anti-PD-1 therapy [58]. Currently, a kind of platinum-based TREM2 inhibitor is under research to deter tumor growth in an MC38-bearing mouse model [72]. Moreover, inhibition of TREM2 downstream molecules such as IL-10 and SYK individually or together has been demonstrated to reverse tumor progression to varying degrees [73]. However, immunotherapy targeted TREM2 needs to be tailored since the role of TREM2 in tumors remains controversial. It is suggested that TREM2 may contribute to tumor suppressing activity in colorectal cancer (CRC) and hepatocellular carcinoma [46-48], while in other cancers such as renal cell carcinoma (RCC) and glioma TREM2 contributes to oncogenic activity [44, 74]. Even within the same cancers, such as hepatocellular carcinoma, TREM2 may play very different or even opposite functions [48, 75]. Therefore, directly targeting TREM2 in tumors where TREM2 plays a tumor suppressor role may lead to resistance or even promote tumor progression. However, most of the emerging literature on the subject of TREM2 consistently reports that TREM2 expression by immune cells creates an immunosuppressive environment that allows the cancer cells to survive better [41, 66, 76]. Considering the multiple functions of TREM2 in different types of myeloid cells, accurately targeting specific types of myeloid cells may avoid the resistance of immunotherapy. For example, targeting TREM2 in macrophages may acquire ideal efficacy since TREM2 can enhance immunosuppressive functions of these cells. However, in NK cells, targeting TREM2 may impede the maturation of NK cells and their ability to kill tumor cells [54]. More related studies are needed to confirm exactly how TREM2 works in tumors, and clinical trials are urgently needed to bring TREM2 to the clinic as soon as possible, thus contributing to new strategies for tumor immunotherapy.


