Introduction
Laparoscopic cholecystectomy is currently the gold standard treatment for gallstone disease and one of the most common general surgical procedures performed worldwide [1–4]. The generally accepted rule of intraoperative visual identification of the cystic duct and the cystic artery during laparoscopic cholecystectomy is the critical view of safety. Nevertheless, bile duct injury (BDI) still occurs at a declining rate of 0.08–0.3% reflecting better equipment, increased experience, and moving beyond the “learning curve” [5–10]. The difficulties in reducing the number of BDI below a certain level force the surgeons to develop new methods of intraoperative visual orientation. Among of them are five B-SAFE landmarks (mnemonic): the Bile duct, the Sulcus of Rouviere, the left hepatic Artery pulsation, the umbilical Fissure, and the duodenum (Enteric) used for surgeon orientation around the gallbladder before dissection in the hepatocystic triangle [3, 11, 12]. Another method that may be used for orientation during laparoscopic cholecystectomy before the critical view of safety was obtained is laparoscopic ultrasound (LUS). LUS is non-invasive, non-irradiating, and may be repeated as many times as it is needed with the possibility of differentiation between vascular and avascular structures [13–15].
Aim
In our study, we are trying to evaluate the use of B-SAFE and ultrasonographic landmarks during laparoscopic cholecystectomy. A comparison of these techniques used in navigation around the gallbladder before dissection in the hepatocystic triangle may help the surgeon to choose the best method to define a safe plane of preparation.
Material and methods
The study group consisted of 158 patients (98 women and 60 men) operated on between April 2018 and March 2020 in one Department of Surgery. The inclusion criterion for the study was symptomatic cholecystolithiasis, which was characterised by episodes of biliary colic and symptoms of chronic cholecystitis. It included pain that was severe and episodic, located in the epigastrium or the right upper quadrant, and lasted 1 to 5 h. It often woke the patient at night or began after a fatty meal. The commonly associated symptoms were nausea and vomiting. Abdominal ultrasound was performed to confirm the diagnosis. Exclusion criteria were pre- or postoperatively diagnosed cancer of the gallbladder (1 patient was excluded due to postoperatively diagnosed cancer of the gallbladder), preoperative acute cholecystitis, and previous operations in the abdominal cavity. Written informed consent was obtained from all patients before surgery. All procedures followed the ethical standards of the 1964 Declaration of Helsinki and its later amendments, and the study was reviewed and approved by the Ethical Committee of the Medical University (approval number BW-24/2020). Cholecystectomies were performed on an elective basis by three surgeons experienced in LC (> 150 cholecystectomies) and LUS (> 70 examinations). For LUS we used a Toshiba PEF-704 LA laparoscopic probe (frequency 7.0–10 MHz) and a Toshiba NemioMX SSA-590A diagnostic ultrasound system, all manufactured in Japan. The LUS probe was inserted through an epigastric 10 mm trocar to obtain the transverse view. The first method of intraoperative orientation during laparoscopic cholecystectomy around the gallbladder was to use the five B-SAFE landmarks. It included the visualisation of any portion of the bile duct, the presence of the sulcus of Rouviere in the form of a “triangle”, “hole”, or “notch” in the liver parenchyma on the right side of the gallbladder, left hepatic artery pulsation in the form of the pulsation on the left side of the porta hepatis, and the presence of an umbilical fissure and the duodenum (Photo 1 A). The presence (or absence) of landmarks was confirmed before any dissection in the hepatocystic triangle after removal of adhesions with the gallbladder and retraction of the gallbladder with the grasper. The second method included the confirmation of three structures of the hepatoduodenal ligament with LUS and colour Doppler function. These were: the bile duct, the proper hepatic artery, and the portal vein, which formed the characteristic MMS (Photos 1 B, 2 A, B). The upper border of MMS was treated in our study as the equivalent of Rouviere’s sulcus (Photo 2 B). The presence of enteric structures was also confirmed with LUS (Photo 2 C). The result of orientation was dissection in the hepatocystic triangle and establishment of the critical view of safety with its three components. It included the clearance of the hepatocystic triangle of all the fibro-fatty and soft alveolar tissue and exposure of the lower third of the cystic plate with only two tubular structures (the cystic duct and the cystic artery) visible entering the gallbladder. The establishment of five B-SAFE landmarks and LUS was attempted routinely in every patient. We set 3 min as the upper time limit to obtain landmarks. Beyond this time we did not search for the landmarks and stated that they were absent.
Photo 1
A – B-SAFE landmarks visible during laparoscopic cholecystectomy. B – LUS is used to obtain ultrasonographic landmarks
A – arterial pulsation, B – bile duct, E – duodenum, F – umbilical fissure, G – gallbladder, S – sulcus of Rouviere, L – liver, LUS – laparoscopic ultrasound.
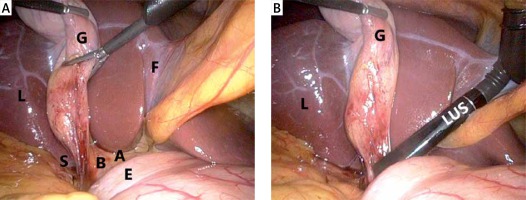
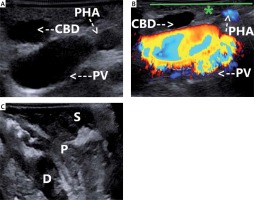
Photo 2
A – “Mickey Mouse” sign-LUS. B – “Mickey Mouse” sign and colour Doppler function; the green line (*) defines the future plane of safe dissection-LUS. C – LUS visualises the enteric structures: the stomach, the pylorus, and the duodenum
CBD – common bile duct, D – duodenum, LUS – laparoscopic ultrasound, P – pylorus, PHA – proper hepatic artery, PV – portal vein, S – stomach.
Results
LUS was significantly more successful in the identification of the upper border of the MMS, the bile duct, and the hepatic artery pulsation in patients with body mass index (BMI) ≥ 30 kg/m2 and fibrosis and chronic inflammation in the gallbladder neck than B-SAFE. LUS was also significantly more successful in the identification of the bile duct in the whole study group than B-SAFE. There were no significant differences between LUS and B-SAFE according to the identification of the upper border of the MMS, Rouviere’s sulcus, and the arterial pulsation in the whole study group. There were also no significant differences according to the identification of duodenum in the whole study group, in patients with BMI ≥ 30 kg/m2 and fibrosis and chronic inflammation in the gallbladder neck (Table I). We did not compare the identification of the umbilical fissure because there is no equivalent of this anatomical structure in LUS. The conversion was performed in the early stage of operation in 2 (1.3%) patients. They had only the umbilical fissure identified (B-SAFE) without visualisation of MMS (LUS) due to undissectable fibrous tissue and chronic inflammation in the region of the gallbladder neck. Due to the undissectable fibrous tissue in the region of the hepatocystic, triangle, conversion was performed in the later stage of operation in the next 2 (1.3%) patients. In both patients all four ultrasonographic landmarks were confirmed, from B-SAFE: in 1 patient there were three landmarks, apart from the sulcus of Rouviere and the bile duct, and in the second patient there were three landmarks, apart from the bile duct and the arterial pulsation. There were no significant differences according to the amount of time needed to obtain B-SAFE and LUS landmarks (3.2% vs. 3.0% of the mean operating time, respectively) (Table I). We did not observe any BDI.
Table I
Characteristics of the study group according to the age, operating time, length of hospital stay after surgery, BMI, number of positive identifications of the umbilical fissure, Rouviere’s sulcus, the bile duct, the arterial pulsation, and the duodenum
Discussion
The main reason for BDI is not the lack of skill, inadequate knowledge, or misjudgement but misperception [16]. The initial false anatomic interpretation or inappropriate orientation before dissection in the hepatocystic triangle may lead to BDI during laparoscopic cholecystectomy [15, 17, 18]. B-SAFE landmarks were developed in response to this problem to help the surgeon in orientation around the gallbladder [3, 12, 19]. Schendel et al. stated that the key landmark to visualise during cholecystectomy is the bile duct, not the gallbladder. Good landmarks in surgery should meet three requirements: they must be present in a high percentage, they must be easy to find and recognise, and their visualisation must be associated with important structures that are going to be dissected [3].
The sulcus of Rouviere is an established landmark for superior/inferior orientation and identification of the plane of the common bile duct; any dissection below the sulcus may result in BDI [3, 20, 21]. There are three basic forms of this landmark, an open triangle being the most common one with the right portal pedicle mostly present on its floor [3, 21]. The prevalence of the Rouviere’s sulcus is 80–90.6% (in our study 71%), with the group of 10–29% of patients where it is absent making the B-SAFE method less reliable [3, 22]. Conversely, the upper border of MMS was identifiable in our study in 99% of patients. Only 2 patients without this landmark were characterised by undissectable advanced chronic inflammation and fibrosis which disabled access to the gallbladder’s neck, leading to prompt conversion. Especially in the group of obese patients (BMI ≥ 30 kg/m2) and in patients with chronic inflammation and fibrosis, LUS enabled significantly better visualisation of the upper border of MMS. Every dissection that is performed over this border is safe and is independent of the traction applied to the gallbladder wall. The landmark moves together with the gallbladder, and its presence can be confirmed in every moment with LUS. Unlike the upper border of MMS, Rouviere’s sulcus is a fixed point, and it is possible that during traction or in the case of chronic inflammation and fibrosis the structures of the hepatoduodenal ligament will be located under this landmark.
Regarding the identification of two other structures of the hepatoduodenal ligament, the bile duct and the arterial pulsation, LUS in our study was significantly more successful than B-SAFE, especially in the group of obese patients (BMI ≥ 30 kg/m2) and in patients with chronic inflammation and fibrosis. Arterial identification in B-SAFE used to fix the left/right position is not direct but we can only assume it from arterial pulsation on the left side of the porta hepatis. The bile duct in obese patients or in case of fibrosis will be obscured through the overlying tissues [3]. LUS bypasses these obstacles because without any additional dissection it enables very precise identification of these structures behind the visible plane. The duodenum is usually not hard to find, and being too close to it means that surgeon is too low and dissection will be close to the common bile duct [3]. In a study by Schendel et al. only 65% of patients had all four landmarks (in our study 68%) visualised in B-SAFE. We did not include the umbilical fissure used for left/right orientation, which is located between the left lateral (segments 2 and 3) and left medial section (segment 4) where the falciform ligament and ligamentum teres lie, because there is no equivalent of this structure in LUS; the dissection should start to the right of this structure [3]. LUS in our study enabled visualisation of 99% of ultrasonographic landmarks in the whole study group, in 100% of patients with BMI ≥ 30 kg/m2, and in 92% of patients with chronic inflammation and fibrosis.
LUS is safe, quick, noninvasive, non-irradiating, and may be performed an infinite number of times whenever it is needed. Intraoperative cholangiography is time-consuming, irradiating, and associated with cystic duct cannulation after dissection in the hepatocystic triangle. Near-infrared fluorescent cholangiography visualises only the bile duct. On the other hand, LUS can be performed before any dissection in the hepatocystic triangle, and it visualises and differentiates the vascular and avascular structures behind the visible plane of future dissection with potential protection against BDI and vasculobiliary injury [13, 15]. The confirmation of ultrasonographic landmarks during LUS may also play an important role in photographic documentation, which may be used for teaching and medicolegal purposes [18]. In comparison to LUS, which is associated with the use of an expensive laparoscopic ultrasound probe, the advantage of B-SAFE seems to be only the cost-free nature of this technique. With reference to the rate of visualisation and quality of the key landmarks, especially in groups where the risk of BDI is higher, its efficacy is significantly lower. Therefore, LUS should be primarily recommended for obese patients with chronic inflammation of the gallbladder as a time-efficient and effective method of navigation around the gallbladder. Patients with “preoperative acute cholecystitis” were excluded from the study because we were trying to have as homogenous a group as possible, operated by the same surgeons experienced in LUS, to obtain high-quality of data. The range of acute cholecystitis is very wide, from mild cases to abscesses, necrosis, perforations, and fistulas to the surrounding organs, making comparative statistical analysis more complicated and necessitating a larger group of patients than in our study. Acute cases are operated usually on duties also by surgeons not experienced in LUS or residents, and time-pressure usually discourages the performance of technically demanding examinations. Of course, B-SAFE may be performed in “acute cholecystitis” cases, but we aimed to compare it with LUS, which in such circumstances is limited due to the above-mentioned reasons.
The recent development of artificial intelligence systems used for navigation during laparoscopic cholecystectomy, which are based on the identification of visual landmarks: the cystic duct, the common bile duct, the lower edge of the left medial liver segment, and the Rouviere’s sulcus, could also be based on ultrasonographic landmarks, which are more common and reliable [23]. Perhaps in the future the laparoscopic view will be connected with the ultrasound probe for better navigation in the hepatocystic triangle.
The limitation of our study was the relatively small number of patients and its single-centre nature. Further studies including larger groups of participants and preoperative acute cholecystitis cases, in more than one surgical centre, are needed to strengthen our findings and confirm the superiority of LUS over B-SAFE, especially in protection against BDI.
Conclusions
Any additional technique that may prevent BDI should be considered among surgeons who perform laparoscopic cholecystectomy. B-SAFE, similarly to the critical view of safety, is only a visual technique, which may be imperfect in some conditions. Conversely, LUS enables effective, functional, and unequivocal confirmation of the safety planes and character of visualised structures associated with the safe laparoscopic cholecystectomy.









