Introduction
Mirizzi syndrome is a type of biliary system obstruction caused by stones impacted in a gallbladder neck or cystic duct situated parallel to the common bile duct, causing extrinsic common bile duct stenosis or obstruction, which can lead to recurrent obstructive jaundice, bile duct erosion, and cholangitis [1–3]. The preoperative diagnosis of Mirizzi syndrome is difficult, and if it is not diagnosed correctly before surgery, there is an elevated risk of intraoperative bile duct injury [4, 5]. Therefore, the preoperative identification and classification of Mirizzi syndrome is vital to a good surgical result [6–8]. Mirizzi syndrome classification is a pathological classification; there is no standard imaging classification. In this study, we explored the applicability of two pathological classification systems to diagnostic imaging.
Material and methods
We reviewed the cases of 76 patients clinically suspected of having Mirizzi syndrome, who were treated in our hospital over almost 5 years (2010–2015), and who had complete clinical and imaging data.
The patients included 31 males and 45 females aged 37–80 years (mean: 56.5). All patients were the same when using different imaging classification. All patients were selected according to the following criteria. (1) Complete clinical and pathological imaging information available. (2) Patients with Mirizzi syndrome confirmed by pathology. (3) Patients with serious bile duct diseases, such as cholangiocarcinoma, were excluded from the study. Of these, 58 patients had had unexplained right upper quadrant pain ranging from 1 week to 2 years. On physical examination, 54 patients had right upper quadrant tenderness and 11 patients had rebound tenderness. Laboratory studies revealed 45 patients with elevated serum γ glutamyl transferase (GGT), 1.2–3.41 μkat/l (normal: 0.13–0.97 μkat/l), 34 patients with hyperbilirubinemia, 25–38 μmol/l (normal: 3.4–20 μmol/l), 33 patients with elevated serum alanine aminotransferase (ALT), 1.12–2.42 μkat/l (normal: 0.08–0.83 μkat/l), 12 patients with elevated alkaline phosphatase, 0.9–2.14 μkat/l (normal: 0.38–0.62 μkat/l), 11 patients with elevated lactate dehydrogenase (LDH), 4.54–6.26 μkat/l (normal: 1.74–4.09 μkat/l), and 9 patients with elevated serum aspartate aminotransferase, 1.09–1.97 μkat/l (normal: 0.08–0.83 μkat/l).
Imaging
A 64-slice spiral CT scanner (Aquilion, Toshiba Medical Systems, Otawara, Japan) was used in this study. The research was performed according to the World Medical Association Declaration of Helsinki. The patients were scanned from the diaphragm to the pubic symphysis. After a noncontrast acquisition, dual-phase enhanced scanning was performed following intravenous injection of 1.5–2.0 ml/kg of iopromide (Ultravist, Schering, Berlin, Germany) with a high-pressure injector at a rate of 2.0–3.0 ml/s (arterial phase delay 20–25 s, portal venous phase delay 60–70 s) using the following settings: 100–120 kV, 150–180 mA, 10 mm section thickness, 10 mm of the layer spacing, pitch = 1.0. After scanning, data were sent to the workstation for three-dimensional reconstruction.
MRI (1.5 T HD Twin Speed General Electric, Milwaukee WI, USA) with an eight- channel phased-array body coil was used to acquire images from the liver dome through the pubic symphysis. First, respiratory-gated and fast spin-echo (FSE) axial T2-weighted images (T2WI) were acquired with a 228 × 224 matrix. Subsequently, MR cholangiopancreatography (MRCP) was performed with 6 mm section thickness and 10 mm layer spacing, followed by breath-hold three-dimensional fast spoiled gradient echo T1WI axial images. In the enhanced scanning, a three-dimensional ultrafast multi-phase enhanced scanning sequence was used. The contrast agent was Gd-DTPA.
3D reconstruction were analyzed by two experienced chief radiologists who were blinded to the pathology results. They solved problems when they were not in agreement. They were good at abdominal imaging diagnosis.
Statistical analysis
Both the Csendes and Nakagawa pathological classification systems were applied to imaging findings, and compared with the pathologic results. We calculated the sensitivity, specificity, accuracy, the rate of missed diagnosis, misdiagnosis rate, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. We compared the correlation (κ consistency test and 95% CI) between each image classification and pathological classification.
Results
Pathological results
All 76 patients underwent open cholecystectomy with choledochobiliary fistula repair, choledochoplasty, or bilioenteric anastomosis performed based on the imaging findings and the surgeons’ experience. Their pathologies were all chronic cholecystitis with cholelithiasis. Under the microscope, gallbladder epithelial atrophy was observed with wall fibrosis and lymphocyte infiltration.
Imaging results
Using the 1989 Csendes classification, computed tomography (CT) and magnetic resonance imaging (MRI) classified 41 patients as type I, 5 patients as type II, 8 patients as type III, and 5 patients as type IV. Seventeen patients were diagnosed with disease other than Mirizzi syndrome. According to the 1997 Nagakawa classification, CT and MRI classified 41 patients as type I, 18 patients as type II, 10 as type III, 4 patients as type IV, and 3 patients without Mirizzi syndrome (Figure 1).
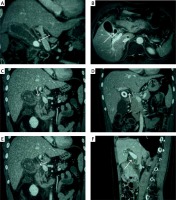
Figure 1
A – CT coronal scan in portal venous phase shows gallbladder neck incarcerated, oppression of common hepatic duct (white arrow). B – MRI contrast-enhanced T1WI shows gallbladder neck incarcerated (white arrow), with the level of hepatic duct stenosis, belonging to Nagakawa type I. C – CT coronal scan in portal venous phase shows gallbladder neck incarcerated (white arrow), with the level of hepatic duct stenosis, belonging to Nagakawa type I. D – CT coronal scan in portal venous phase shows gallbladder and common hepatic duct fistula (white arrow), with 2/3 of the fistula over the circumference of the biliary tract, belonging to Nagakawa type II. E – CT coronal scan in portal venous phase shows stone located in the confluence of cystic duct and common bile duct (white arrow), belonging to Nagakawa type III. F – CT sagittal scan in portal venous phase shows no stone in cystic duct and gallbladder neck (white arrow), with inflammatory stricture of common bile duct, belonging to Nagakawa type IV
Pathological classification
According to the 1989 Csendes classification, CT and MRI classified 36 patients as type I, 16 patients as type II, 4 patients as type III, 1 patient as type IV, and 19 as another diagnosis.
According to the 1997 Nagakawa classification, CT and MRI classified 36 patients as type I, 21 patients as type II, 12 patients as type III, 5 patients as type IV, and 2 patients as another diagnosis.
Statistical evaluation
The accuracy analysis of the two pathology classification systems was evaluated. They only have differences in positive predictive value and positive likelihood ratio (Table I), suggesting that both pathology classification systems have similar results for imaging diagnosis. The correlation between imaging and pathological classifications was evaluated.
Table I
The accuracy analysis of the two pathology classification systems in these patients
According to the Csendes classification as ascertained by imaging, 41 cases were type I, 5 were type II, 8 were type III, 5 were type IV, and 17 had another diagnosis.
According to findings in pathology, there were 36 type I patients, 16 type II, 4 type III, 1 type IV, and 19 had another diagnosis. The κ consistency examination on image classification and pathological classification was done and κ = 0.57 (Table II), suggesting that the correlation was acceptable. According to the Nagakawa classification as ascertained by imaging, 41 cases were type I, 18 were type II, 10 were type III, 4 were type IV, and 3 had another diagnosis. According to the pathological classification, there were 36 type I patients, 21 type II, 12 type III, 5 type IV, and 2 had another diagnosis. The κ consistency test on image classification and pathological classification showed κ = 0.68 (Table III), suggesting a good correlation. Ridit analysis (95% confidence interval) shows that Csendes pathology classification and Nagakawa pathology classification, Csendes pathology classification and Csendes imaging classification, and Nagakawa pathology classification and Nagakawa imaging classification have no significant statistical difference (p > 0.05) (Tables I, IV).
Table II
Consistency between image classification and pathological classification (Csendes classification)
| Pathology image | I | II | III | IV | None | Total |
|---|---|---|---|---|---|---|
| I | 31 | 10 | 0 | 0 | 0 | 41 |
| II | 1 | 4 | 0 | 0 | 0 | 5 |
| III | 3 | 0 | 3 | 0 | 2 | 8 |
| IV | 0 | 1 | 1 | 1 | 2 | 5 |
| None | 1 | 1 | 0 | 0 | 15 | 17 |
| Total | 36 | 16 | 4 | 1 | 19 | 76 |
Table III
Consistency between image classification and pathological classification (Nagakawa classification)
| Pathology image | I | II | III | IV | None | Total |
|---|---|---|---|---|---|---|
| I | 31 | 7 | 3 | 0 | 0 | 41 |
| II | 3 | 14 | 0 | 1 | 0 | 18 |
| III | 1 | 0 | 9 | 0 | 0 | 10 |
| IV | 0 | 0 | 0 | 4 | 0 | 4 |
| None | 1 | 0 | 0 | 0 | 2 | 3 |
| Total | 36 | 21 | 12 | 5 | 2 | 76 |
Table IV
Ridit analysis (95% CI)
Discussion
Mirizzi syndrome is extraneous compression of the common bile duct by a stone or stones in the gallbladder neck or in an anatomically variant cystic duct situated parallel to the common duct. The first reported case dates back to the early 20th century [9] and was named after the Argentinean surgeon Mirizzi in 1948.
Preoperative diagnosis of Mirizzi syndrome is difficult, and intraoperative bile duct injury can easily occur in unsuspected cases, which can cause serious complications. Preoperative imaging can help the surgeon to avoid confusing the common bile duct with the parallel cystic duct.
The surgical treatment of Mirizzi syndrome is related to its type [10–12]. In 1989, Csendes et al. [13] divided Mirizzi syndrome into four types according to the degree of common bile duct damage, in which type I featured extrinsic common bile duct compression by stones in the cystic duct or gallbladder neck; type II was further complicated by a cholecystobiliary fistula involving less than 1/3 of the common bile duct circumference; type III featured a fistula involving up to 2/3 of-the common bile duct circumference; and type IV featured complete destruction of the common bile duct. In 1997, Nagakawa et al. [14] proposed a new classification method, in which type I again featured extrinsic common bile duct compression by stones in the cystic duct or gallbladder neck; type II featured a cholecystobiliary fistula; type III had stones located at the confluence of the cystic duct and common hepatic duct; and type IV had no stones in the cystic duct or gallbladder neck, but featured inflammation around a common hepatic duct stricture induced by compression by cholecystitis with or without stones in the gallbladder. In the Nagakawa classification, Csendes types II–IV correspond to Nakagawa type II, and types III and IV are exclusive to the Nakagawa classification.
These classifications of Mirizzi syndrome are pathological, and the preoperative classification of Mirizzi syndrome can only rely on diagnostic imaging. As ultrasound often cannot visualize the common bile duct, Mirizzi syndrome assessment is mainly dependent on CT and MRI examinations [15]. We believe it is for the following reasons: the Csendes classification focuses on the measurement of fistula size, but it is difficult to estimate the amount of common bile duct circumferential involvement on CT or MRCP. The Nagakawa classification does not require quantification of bile duct fistula involvement, but only requires ruling in or out a cholecystobiliary fistula, assessing biliary stones, and detecting common bile duct inflammatory strictures, which are well within the capabilities of CT and MRI/MRCP. The Nagakawa classification was higher in sensitivity, specificity, accuracy, positive predictive value, positive likelihood ratio, Youden index, with lower rates of both missed diagnosis and misdiagnosis. There was a significant difference in positive predictive value and positive likelihood ratio in favor of the Nagakawa system. The κ consistency test showed that the Nagakawa classification had a better correlation between imaging classification and pathological classification though Ridit analysis (95% CI) shows no statistically significant difference.
In conclusion, in this study, imaging was used as an approach to simulated preoperative pathological classification, based on the Csendes and Nagakawa classification systems. The results of this study showed that, compared with the Csendes classification system, the Nagakawa classification system was superior to the Csendes system in many statistical indexes for diagnostic accuracy, especially as the consistency between Csendes imaging classification and pathological classification was low. Compared with the Csendes classification, the Nagakawa classification is more suitable for image-based preoperative planning.


