Introduction
Hypersensitivity pneumonitis (HP) is an interstitial lung disease caused by exaggerated immune response to inhalation of various organic antigens. HP constitutes the third most common interstitial lung disease (after idiopathic lung fibrosis and interstitial lung disease in the course of collagen tissue disease) [1, 2]. The annual incidence rate of HP is estimated at 0.9-1.8 per 100,000 [1, 3, 4]. Clinical diagnostic algorithm of HP, proposed recently by Vasakova et al. [5], takes into account three diagnostic criteria:
Signs and symptoms of pulmonary disease in the persons with repeated occupational or environmental exposure to specific, mostly organic, antigens, +/- the presence of serum specific IgG antibodies.
Radiological signs of interstitial lung disease (ILD) described on high resolution chest computed tomography (HRCT), with characteristic features such as: ill-defined centrilobular nodules, mosaic lung attenuation composed of areas of ground glass opacities and air-trapping, and the predominance of attenuations in the upper parts of the lungs [6].
Lymphocytosis in bronchoalveolar lavage (BAL), exceeding 30%, in most patients [7, 8].
Lung biopsy, for confirmation of HP, is suggested in patients who do not fulfil the above mentioned criteria. The histologic features of HP consist of inflammatory lymphocytoplasmatic infiltrations with bronchiolocentric distribution, the presence of poorly formed granulomas, and negative cultures for acid-fast bacilli and fungi [9].
Specific serum IgG antibodies (ssIgG) to various avian proteins, bacterial compounds, or molds may be assessed in serum or BAL in the HP diagnostic pathway [10]. Precipitation, Ouchterlony double diffusion, or immunoelectrophoresis are used for qualitative determination of antibodies [10]. ELISA, Immun<sup>o</sup>CAP and Immulite tests enable quantitative determination of ssIgG [10].
The aim of the present retrospective study was to assess the results of ssIgG evaluation in HP patients and their correlation with clinical data.
Material and methods
128 patients, 65 females, 63 males, median age 53 years (range, 18-75 years), with HP recognized in the period of 2005-2015 participated in the present study. The diagnosis of HP was established based on clinical data concerning the exposition to organic allergens, symptoms of disease developing as a result of contact with allergen, characteristic pattern of changes in HRCT, and lymphocytosis in BAL exceeding 30%. The data concerning the exposition was documented in medical history of patient as a result of face to face interview, and collected retrospectively by the authors. In case of diagnostic difficulties, the lack of exposition, non-characteristic HRCT pattern, or lack of lymphocytosis in BAL, lung biopsy was performed. The details of HP diagnostic pathway in the presented group of patients, have been described previously [11].
The control group consisted of 102 patients, 61 males, 39 females, median age 65 years (range, 23-95 years), with various interstitial lung diseases, other than HP: 33 with idiopathic pulmonary fibrosis, 17 with ILD in the course of collagen tissue disease, 13 with sarcoidosis, 7 with non-specific interstitial pneumonia, 6 with cryptogenic organizing pneumonia, 5 with desquamative interstitial pneumonia, 2 with respiratory bronchiolitis interstitial lung disease, and 19 with chronic fibrotic lung disease not classified.
Patients’ sera were obtained before the treatment. The tests were performed on fresh sera stored for maximum of 4 days at 2-8°C. The study included antigens of thermophilic actinomycetes and protein antigens from birds’ droppings prepared in our laboratory for internal use and validated in the past.
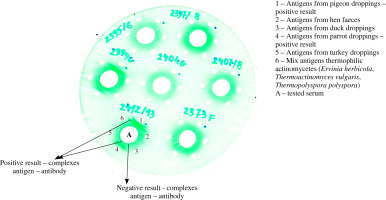
Assessment of antibodies to various organic antigens was performed by double diffusion in agar gel using Ouchterlony method. Antigens of thermophilic actinomycetes (Ervinia herbicola, Thermoactinomyces vulgaris, Thermopolyspora polyspora) and protein antigens from birds’ droppings (pigeons, hens, ducks, parrots, turkeys) were applied to separate wells on agar gel located at a distance of 1 cm from the central well containing the tested serum. The plates were incubated for 5-7 days at 27°C, then washed in physiological saline solution for 6 days. Next, the agar gels were dried at room temperature for 48 hours and stained in 0.1% light green solution. The test results were read after 24 hours: positive result – visible precipitation lines (antigen-antibody complexes), negative result – no precipitation lines (Fig. 1). The results of precipitins serology were compared with the data concerning exposition.
Fig. 1
The results of precipitation test with thermophilic actinomycetes and with protein antigens of birds’ droppings
Statistical analysis: The variables were presented as numbers or percentages of positive results. Chi-square test was used to compare the number of positive results in different groups of patients.
The publication of the results of the study was approved by the Bioethical Committee, National Research Institute of Tuberculosis and Lung Diseases, No 14/2019.
Results
Medical history confirmed the exposition to various birds’ allergens in 77/128 (60%) of HP patients. Exposure to pigeons was reported by 29 patients: 15 pigeon breeders, 9 families of pigeon breeders, 3 nearest neighbors of pigeon breeders, 2 persons working on reconstructions of roofs and attics. Exposure to parrots (10 patients) concerned the parrot home breeders.
Occupational exposure to poultry proteins was documented in 54 patients living in the farms and breeding the different types of poultry. No data concerning the number of birds in the flocks were available. The data concerning feather duvet or down jackets have not been reported by our patients.
Positive precipitins to any of above mentioned organic antigens were obtained in 73 out of 128 of all HP patients (57%) and in 61% of those exposed to such antigens (Table 1). Positive results in the control group were obtained in 7 out of 102 patients (7%). Sensitivity of ssIgG in the whole HP group was 0.57, in the persons with positive exposure 0.61, and with specificity 0.93.
Table 1
Results of precipitins measurement in 128 HP patients comparing to control group of patients with other interstitial lung diseases (102 patients)
Precipitins to at least one bird antigen was confirmed in 49 out of 77 HP patients exposed to birds (64%) (Table 2). The percentage of cases with serologic confirmation of ssIgG presence was significantly higher in case of contact with parrots of pigeons (80% and 76%, respectively) comparing to poultry (54%), p = 0.02. However, the accordance rates between specific precipitins to parrots, pigeons, and poultry, and the exposition to those species were very similar (40%, 48%, and 44%, respectively). Positive precipitins despite unproved exposure to birds were found in 35% of HP patients ( mostly to multiple birds’ species).
Table 2
Results of precipitins measurement according to declared exposure to various birds’ species in 128 hypersensitivity pneumonitis patients
The exposition to hay or hay products was confirmed in 63 out of 128 patients (49%), significantly more frequently in males comparing to females, p = 0.0015 (Table 3). Positive precipitins to thermophilic bacteria were found in 29% of exposed patients only (Table 4).
Table 3
Exposure to hay and hay products according to gender in 128 hypersensitivity pneumonitis patients
| Gender | n | Positive exposure n (%) | Negative exposure n (%) |
|---|---|---|---|
| Males | 63 | 40 (64)* | 23 (36) |
| Females | 65 | 23 (35)** | 42 (65) |
| Total | 128 | 63 (49) | 65 (51) |
Table 4
Results of precipitins to thermophilic bacteria according to declared exposure to hay or hay products in 128 hypersensitivity pneumonitis patients
| Exposure | n | Positive precipitins n (%) | Negative precipitins n (%) |
|---|---|---|---|
| Positive | 63 | 18 (29) | 45 (71) |
| Negative | 65 | 4 (6) | 61 (94) |
| Total | 128 | 22 (17) | 106 (83) |
Precipitins to both birds antigens and thermophilic bacteria were found in 16 patients, among them 9 declared exposure to both avian proteins and hay compounds, 6 patients declared exposure to hay compounds, and 1 declared the exposure to avian proteins.
Discussion
According to published recommendations, the most important problem in HP diagnostics is the confirmation of contact with organic allergens [5, 8]. We confirmed the exposition to birds’ antigens in 60% of HP patients, to hay or hay products in 49%, and to any of above mentioned antigens in 77% of patients. These data are in agreement with observations from other studies where birds’ antigens were responsible for the most HP cases in their series [12, 13].
The role of precipitins to specific organic antigens in the diagnostic algorithm of HP is still under debate [14, 15]. Positive precipitins are found in 40-60% of exposed healthy persons, indicating the immunization state [10, 14, 16]. The development of immune complexes mediated lung disease, with various degree of fibrosis, depends probably on many interfering factors as well as genetic predisposition [17].
Therefore, the role of ssIgG in HP diagnostic algorithm should be investigated only in patients with clinical and radiological signs of HP.
The present study revealed the presence of ssIgG in 57% of all HP patients, and in 61% of those exposed to birds’ dropping and/or hay and hay products. In the control group of patients with ILDs other than HP, positive results of ssIgG were found in 7%. Thus, the sensitivity of precipitins in the whole group was 0.57 and in the exposed group 0.61, with the specificity of 0.93 comparing to non-HP ILDs.
The results of other authors’ indicate that sensitivity of ssIgGs in HP may depend on clinical characteristics of the patients and the methods used. Suhara et al. documented ssIgG sensitivity of 0.8-1.0 in acute bird-related HP, and 0.26-0.79 in chronic bird-related HP [18]. Simpson et al. reported that ELISA test showed greater sensitivity comparing to double immunodiffusion method [19]. Specificity of the test depends on the type of population included in the control group. The results obtained with the same type of assay as used in our study, in healthy persons not exposed to pigeons, revealed 9% of positive ssIgG against pigeons droppings [20]. Recently, Giaconi et al. investigated 108 patients with chronic fibrosing ILD and found 0.68 specificity of ssIgG for the recognition of HP [21].
In our study group, an important observation concerned patients with unproved contact with birds’ allergens in whom ssIgG were positive (35%) mostly to poultry and pigeons. According to other studies, ssIgG presence in HP patients denying the contact with birds’ allergens may serve as a proof of exposition [22-24]. One of neglected by patients forms of exposition to birds’ allergens is the contact with feather filling of pillows and clothing (feather duvet lung) [25]. The data concerning feather duvet or down jackets have not been reported by our patients; however, this type of information could be missed due to retrospective character of data collection.
We did not confirm the allergy to thermophilic actinomycetes in most of our patients who reported the exposition to hay or hay products. The data from literature indicate that farmer’s lung may be caused either by thermophilic bacteria or by molds [10, 24, 26, 27]. Molds can induce farmer’s lung in large agriculture regions, especially during wet and warm seasons [24]. Most frequently involved are Alternaria, Aspergillus, and Botrytis species [24], and in some geographic regions, Absidia (Lichtheimia corymbifera) and Wallemia were suggested as the cause of farmer’s lung disease [26, 27]. As molds’ antigens were not included in our laboratory set, this type of allergy could be missed out in our material.
Identification and eradication from patient’s environment, the antigen responsible for HP pathogenesis, is essential for the treatment and management of HP. The presence of precipitins to specific organic antigens indicate dependable contact of the patients with certain organic substances and enables their elimination from the environment.
Conclusions
Positive precipitins were obtained in 57% of all HP patients and in 61% of those exposed to above mentioned antigens. Positive results in the control group were obtained in 7% of patients. Sensitivity of ssIgG in HP group were 0.57, and specificity 0.93.
Positive serology to birds’ antigens was confirmed in 64% of HP patients who have the contact with birds, significantly more often in those exposed to parrots and pigeons comparing to those exposed to poultry.
The comparison of ssIgG with exposition data indicated the possibility of cross reactivity between various avian antigens.
Positive avian serology was confirmed in 35% of HP patients with unproved exposure to birds antigens; this information might be important in HP diagnostic algorithm and management of HP.
Precipitins to thermophilic bacteria were found in 29% of HP patients exposed to hay or hay products. Low-rate of confirmation of farmer’s lung with precipitins in our material may be caused by lack of mold antigens in our testing probes.


