Introduction
Systemic lupus erythematosus (SLE) is a spectrum of autoimmune disease in which defects can occur at multiple points of the immune cascade [1]. At present, SLE is recognized with 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACE) criteria [2]. In addition, SLE can lead to a striking heterogeneity of clinical presentations [3]. The central pathology is the recognition of, and reaction to, self-antigens by the immune system, leading to type I interferon signaling and the production of pathogenic auto-antibodies, which mediate much of the disease [2, 4]. Lupus nephritis (LN) is one of the most serious manifestations of SLE and is a crucial predictor of poor outcome [5], which is classified with revised International Society of Nephrology criteria [4]. Occurrence and progression of LN are the end result of complex interactions between regulation of immune responses and the pathological process involving renal resident cells [6, 7]. Moreover, the progression of LN may lead to the occurrence of renal failure [8]. Nowadays, the main treatment of LN includes glucocorticoid and immunosuppressive therapy [9]. However, the therapeutic effect is still not ideal. Thereby, it is necessary to find new strategies for the treatment of LN.
T cell immunoglobulin and mucin 1 (Tim-1) is an important gene that mediates T-helper cell development [10]. Tim-1 is expressed in CD4+ T cells and starts transcription at the initial stage of antigen stimulation, which provides a costimulatory signal for T cell activation, participates in T cell proliferation and differentiation, and inhibits the occurrence of peripheral tolerance [11-13]. These findings suggest that Tim-1 is a key gene that can regulate T cells and is likely to be an immune marker. Meanwhile, Tim-1 has been proved to inhibit the progression of LN [14, 15]. However, the regulatory role of Tim-1 in LN is largely unknown.
Autophagy stands for an ability of cells to prevent their own death [16], which could also protect cells against apoptosis [17]. In addition, some reports have proved that autophagy can be an important mediator in the immune system [18, 19]. Meanwhile, autophagy is closely associated with progression of SLE/LN. For instance, autophagy may contribute to the progression of SLE [20]. Yang et al. reported that autophagy can promote the occurrence of LN [21]. Thus, autophagy plays a key role in LN. It has been previously reported that Tim-1 is closely related with autophagy. For example, Tim-1 may participate in the activation of Dengue virus-induced autophagy [22]. Tim-1-mediated phagocytosis can link ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation [23]. However, the association between Tim-1 and autophagy in LN remains unclear.
In the current study, we sought to investigate the function of Tim-1 in LN in vitro. We investigated the function of Tim-1 in LN in vitro as well as the correlation between Tim-1 and autophagy in LN. We hope our research will provide a new strategy for the treatment of LN, as well as a new hope for patients with LN.
Material and methods
Cell culture and treatment
Human immortalized podocytes were obtained from American Type Culture Collection and cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 2 mM glutamine (Sigma, St. Louis, MA, USA) at 37°C. Podocytes were treated with immunoglobulin G (IgG; Sigma) for 24 h to establish an in vitro model of LN. To confirm the correlation between Tim-1 and autophagy in LN, podocytes were treated with an autophagy inhibitor (3-methyladenine and bafilomycin A1, Sigma).
Cell transfection
Tim-1 overexpression (pcDNA-3.1-Tim-1) and empty vector (pcDNA3.1) were purchased from Genepharma (Shanghai, China), and were transfected into podocytes using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions.
In vivo study
Ten female MRLlpr/lpr lupus prone mice and 5 control female C57BL mice were acquired from Nanjing Medical University. C57BL mice served as control mice. In addition, MRLlpr/lpr lupus prone mice were randomly divided into 2 groups: MRLlpr/lpr and LN. Mice in the control and MRLlpr/lpr groups were anesthetized by an intraperitoneal injection of 2% pentobarbital sodium at a dose of 45 mg/kg body weight at the age of 3 months, while mice in the LN group were injected at the age of 6 months. Kidney tissues were collected from mice and harvested. All the procedures were approved by the Institutional Animal Care and Use Committee of the local hospital.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay was used to assess cell proliferation according to the manufacturer’s protocol. Podocytes were plated (5 × 103 cells/well) into 96-well plates and treated for 24, 48 or 72 h at 37°C as follows: Tim-1, IgG or Tim-1 + IgG. Subsequently, podocytes were incubated with 10 µl of CCK-8 reagents (Beyotime, Shanghai, China) for 2 h at 37°C. The absorbance of each well was measured at a wavelength of 450 nm using a microplate reader (Thermo Fisher Scientific).
Cell apoptosis analysis
Podocytes were seeded in a 6-well plate (5 × 104 per well). Cells were centrifuged at 956 g for 5 min and the residue was resuspended with 100 µl of binding buffer. Subsequently, 5 µl of Annexin V-FITC and 5 µl of propidium iodide were added to the cells for 15 min at 4°C. Apoptotic cells were analyzed by flow cytometry using a flow cytometer (Becton, Dickinson and Company, USA).
ELISA
The levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor α (TNF-α) in supernatants of podocytes or serum of mice were detected using an ELISA kit (Multisciences (Lianke) Biotech Co., Ltd, Hangzhou, China) according to the manufacturer’s instructions.
Reverse transcription-quantitative PCR
Total RNA was extracted from cell lines or tissues using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Total RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit (Takara, Tokyo, Japan). Subsequently, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed using the SYBR premix Ex Taq II kit (Takara, Tokyo, Japan). Real-time qPCRs were performed in triplicate under the following protocol: 2 minutes at 94°C, followed by 35 cycles (30 s at 94°C and 45 s at 55°C). The following primer pairs were used for RT-qPCR: Tim-1 forward, 5'-GGAAGTGGTGGCTATGAGTCAG-3' and reverse, 5'-TGTCAATTTGAAACTTAAAAAGCAG-3'; IL-6 forward, 5'-GGAAGTGGTGGCTATGAGTCAG-3' and reverse, 5'-TGTCAATTTGAAACTTAAAAAGCAG-3'; IL-1β forward, 5'-GGAAGTGGTGGCTATGAGTCAG-3' and reverse, 5'-TGTCAATTTGAAACTTAAAAAGCAG-3'; TNF-α forward, 5'-GGAAGTGGTGGCTATGAGTCAG-3' and reverse, 5'-TGTCAATTTGAAACTTAAAAAGCAG-3'; β-actin forward, 5'-GTCCACCGCAAATGCTTCTA-3' and reverse, 5'-TGCTGTCACCTTCACCGTTC-3'. The gene levels were quantified using the 2-△△ct method and β-actin was used as an internal reference.
Western blotting
Total protein was extracted from cell lines or tissues using RIPA lysis buffer (Beyotime, Shanghai, China). Total protein was quantified using the bicinchoninic acid protein kit (Thermo Fisher Scientific). Protein (40 µg per lane) was separated with 10% SDS-PAGE and transferred onto PVDF membranes (Thermo Fisher Scientific). Subsequently, the membranes were blocked with 5% skimmed milk in TBST for 1 h at room temperature. The membranes were incubated overnight at 4°C with primary antibodies against: IL-6 (Abcam; ab239007, 1 : 1000), IL-1β (Abcam; ab118973, 1 : 1000), TNF-α (Abcam; ab219620, 1 : 1000), LC3 (Abcam; ab136668, 1 : 1000), p62 (Abcam; ab109330, 1 : 1000), Tim-1 (Abcam; ab5666, 1 : 1000) and GAPDH (Abcam; ab179467, 1 : 1000). Then, the membranes were incubated with HRP-conjugated secondary antibodies (Abcam; ab7356, 1 : 5000) for 1 h. Protein bands were visualized using the ECL kit (Thermo Fisher Scientific). β-actin was used as the loading control. IPP 6.0 (Image-Pro Plus 6.0) was used for the densitometry analysis.
Statistical analysis
Data are presented as the mean ± standard deviation. Comparisons between two groups were analyzed by Student’s t-test. Comparisons among multiple groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test (GraphPad Prism; version 7; GraphPad Software, Inc.). P < 0.05 was considered to indicate a statistically significant difference.
Results
Lupus nephritis mice show high Tim-1expression, strong autophagy and an inflammatory reaction
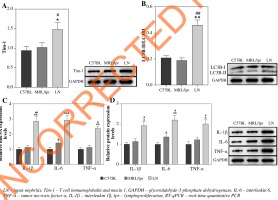
RT-qPCR was performed to detect the expression of Tim-1 in LN mice. As shown in Figure 1A, the expression of Tim-1 was significantly upregulated in LN mice, compared with that in C57BL/6 or MRLlpr/lpr lupus prone mice (p < 0.05). Meanwhile, the ratio of LC3B-II/LC3B-I in LN mice was much higher than that of C57BL/6 or MRLlpr/lpr lupus prone mice (p < 0.01, Fig. 1B). A similar result was also observed in the expression of IL-6, IL-1β and TNF-α at both mRNA and protein levels (p < 0.05, p < 0.01, p < 0.001, Fig. 1C, D). These findings indicated that inflammatory responses were activated in LN and then induced autophagy. Taken together, the above data suggested that LN mice have increased Tim-1 expression, autophagy, and an inflammatory reaction.
Fig. 1
LN mice show high Tim-1 expression, strong autophagy and an inflammatory reaction. A) Expression of Tim-1 in kidney tissues of mice was detected by western blot. The relative protein expression was quantified by normalizing to GAPDH. B) Expression of LC3B in kidney tissues of mice was detected by western blot. The ratio of LC3B-II/ LC3B-I was calculated by normalizing to GAPDH. C) Expression of IL-6, IL-1β and TNF-α in kidney tissues of mice was detected by RT-qPCR. D) Expression of IL-6, IL-1β and TNF-α in kidney tissues of mice was detected by western blot. Relative protein expression was quantified by normalizing to GAPDH. *p < 0.05, **p < 0.01, ***p < 0.001 com- pared to control. #p < 0.05, ##p < 0.01 compared to MRL/lpr
Tim-1 promotes autophagy and alleviates inflammatory response in lupus nephritis cell model
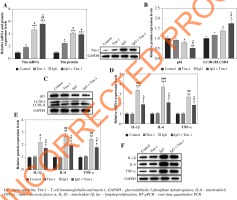
Based on the increased Tim-1 expression in LN mice, we speculated that Tim-1 was involved in the regulation of autophagy and the inflammatory response in the LN cell model. To verify this, we first determined the expression of Tim-1 based on RT-qPCR and western blot. As revealed in Figure 2A, the expression of Tim-1 was notably upregulated in IgG-treated podocytes at both mRNA and protein levels (p < 0.001), and Tim-1 overexpression significantly promoted this trend (p < 0.05). Compared with the control cells, no significant effect was found on the expression of autophagy-related proteins in Tim-1-transfected cells. However, compared with the IgG-treated cells, IgG plus Tim-1 resulted in enhancement of autophagy, as showed by the remarkably decreased p62 and the significantly decreased LC3B-II/LC3B-I ratio (p < 0.05, Fig. 2B, C). Moreover, Tim-1 greatly alleviated IgG-induced increase of IL-6, IL-1β and TNF-α levels in podocytes at both mRNA and protein levels (p < 0.05, Fig. 2D-F). Altogether, Tim-1 enhanced auto-phagy and alleviated inflammatory responses in IgG-induced podocytes.
Fig. 2
Tim-1 promotes autophagy and alleviates inflammatory response in LN cell model. Podocytes were treated with IgG, Tim-1 or Tim-1 + IgG. A) Expression of Tim-1 in podocytes was detected by RT-qPCR and western blot. Relative protein expression was quantified by normalizing to GAPDH. B, C) Protein expression of p62 and LC3B in podocytes was detected by western blot. The ratio of LC3B-II/LC3B-I and the relative expression of p62 were calculated by nor- malizing to GAPDH. D) Expression of IL-6, IL-1β and TNF-α in podocytes was detected by RT-qPCR. E, F) Protein expression of IL-6, IL-1β and TNF-α in podocytes was detected by western blot. Relative protein expression levels were quantified by normalizing to GAPDH. *p < 0.05, **p < 0.01, ***p < 0.001 compared to control. #p < 0.05, ##p < 0.05, ###p < 0.05 compared to Tim-1. ^p < 0.05 compared to IgG
Tim-1 promotes proliferation and inhibits apoptosis in lupus nephritis cell model
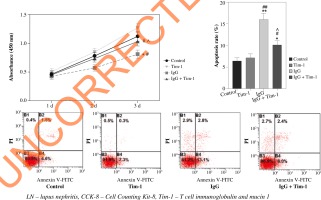
In order to detect the effect of Tim-1 on the proliferation of LN cells, the CCK-8 assay was performed. As revealed in Figure 3A, much lower cell viability was observed in LN cells when compared to the control, and Tim-1 partially reversed this trend (p < 0.05). Meanwhile, limited apoptosis in podocytes was found after IgG treatment, while Tim-1 notably inhibited IgG-induced podocyte apoptosis (p < 0.05, Fig. 3B, C). To sum up, IgG-induced podocyte growth inhibition and apoptosis promotion can be significantly restored by Tim-1.
Fig. 3
Tim-1 promotes proliferation and inhibits apoptosis in LN cell model. A) After 24, 48 or 72 h of incubation, via- bility of podocytes was tested by CCK-8 assay. B, C) Apoptosis of podocytes was tested by flow cytometry. *p < 0.05, **p < 0.01 compared to control. #p < 0.05, ##p < 0.01 compared to Tim-1. ^p < 0.05 compared to IgG
Tim-1 alleviates the inflammatory response of lupus nephritis podocytes via inducing autophagy
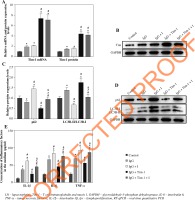
To further confirm the correlation between Tim-1 and autophagy in IgG-treated podocytes, we detected the effect of autophagy inhibitor (I) on Tim-1-mediated autophagy and inflammatory responses. As demonstrated in Figure 4A, B, the IgG-induced increase of Tim-1 expression was greatly enhanced by Tim-1 overexpression, while autophagy inhibitor did not affect Tim-1 expression at either the mRNA or protein level (p > 0.05). However, the inhibitor obviously suppressed the Tim-1 induced enhancement of autophagy in the LN cell model, as shown in the inhibition of expression of p62 and the increase of LC3B-II/LC3B-I ratio (p < 0.05, Fig. 4C, D). Similar, the inhibitory effect of Tim-1 on IgG-induced inflammatory responses in podocytes was also partially offset by autophagy inhibitor (p < 0.05, Fig. 4E). In summary, Tim-1 significantly alleviated the inflammatory reaction of LN cells via inducing autophagy.
Fig. 4
Tim-1 alleviates the inflammatory response of LN podocytes via inducing autophagy. Podocytes were treated with IgG, IgG + autophagy inhibitor (I), Tim-1 + IgG or Tim-1 + IgG + autophagy inhibitor (I). A) Expression of Tim-1 in podocytes was detected by RT-qPCR. B) Expression of Tim-1 in podocytes was detected by western blot. Relative protein expression was quantified by normalizing to GAPDH. C, D) Protein expression of LC3B and p62 in podocytes was detected by western blot. The ratio of LC3B-II/LC3B-I and the relative expression of p62 were calculated by normal- izing to GAPDH. E) Levels of IL-6, IL-1β and TNF-α in supernatants of podocytes were detected by ELISA. *p < 0.05 compared to control. #p < 0.05 compared to Tim-1. ^p < 0.05 compared to IgG + I. &p < 0.05 compared to IgG + Tim-1
Discussion
Systemic lupus erythematosus is a prototypic autoimmune disease characterized by production of a diverse array of autoantibodies, complement activation, and deposition of immune complexes, causing tissue and organ damage [15, 24]. Many patients with SLE develop kidney disease, and LN is one of its most severe manifestations [14]. The immunologic features of LN contain autoantibodies directed against nuclear components and elevated pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α and BAFF [25]. Clinical interventions targeting multiple mediators such as IL-1β, IL-6, TNF-α or BAFF have a very limited effect on LN [26], suggesting that other effector pathways may play a more dominant role in LN pathogenesis.
It has been reported that Tim-1 is involved in immune-related diseases. For example, Tim-1 can mediate CD19+CD24+CD38+ B cell function in primary biliary cholangitis [27]. Chiou et al. stated that Tim-1 plays a key role in the neuro-immune axis [28]. In addition, Tim-1 has been confirmed to be a mediator in immune function regulation [29]. Meanwhile, Tim-1 is known to participate in SLE and LN [15, 30]. In our study, Tim-1 was upregulated in LN, which was consistent with these previous reports. On the other hand, some studies indicated that only the G allele of Tim-1 at the -1454G/A site is a susceptibility variant associated with SLE [14, 31]. Based on these reports, it might be concluded that Tim-1 was downregulated in SLE due to the mutation of the -1454G/A site.
In addition, inflammatory responses and autophagy are involved in progression of LN [9, 32]. Wu et al. found that activation of autophagy may lead to the alleviation of LN severity [33]. Katsuyama et al. reported that immune responses are closely related to the development of LN [34]. Meanwhile, our data suggested that LN in mice is associated with autophagy and the inflammatory reaction. Thus, all these data suggested that Tim-1 alleviated the progression of LN via inhibiting immune responses and activating autophagy. Nevertheless, according to Zhou et al. autophagy could be negatively associated with podocyte injury during the progression of lupus nephritis [35]. Our research was similar to Zhou et al., suggesting that autophagy might inhibit lupus nephritis progression.
To further verify the correlation among Tim-1, autophagy and the immune reaction, podocytes were treated with IgG to mimic LN in vitro. The results showed that Tim-1 could inhibit the expression of IL-6, TNF-α, IL-1β, and LC3II/LC3I ratio and increase the p62 level in IgG-treated podocytes. Consistently, previous reports confirmed the role of immune responses and autophagy in LN [36, 37]. Thus, it could be suggested that Tim-1 could act as an inhibitor in immune responses, as well as a promoter in autophagy.
Based on the above data, we sought to further explore the effect of Tim-1 on growth of IgG-treated podocytes. Thus, flow cytometry and the CCK-8 assay were performed. Our findings demonstrated that Tim-1 could reverse IgG-induced podocyte growth inhibition. In contrast, Zheng et al. found that Tim-1 knockdown could inhibit the apoptosis of neurons in cerebral ischemia-reperfusion injury [38]. The discrepancy might due to different type of diseases.
To confirm the correlation between Tim-1 and autophagy in LN, podocytes were treated with an autophagy inhibitor. The data revealed that the autophagy inhibitor could reverse Tim-1-induced activation of autophagy in LN. Autophagy has been regarded as an evolutionarily process by which eukaryotic cells maintain intracellular homeostasis and adapt themselves to cellular stress to survive. The increase of autophagy activities often leads to inhibition of apoptosis [39]. Consistent with this background, our finding suggested that Tim-1 inhibited the apoptosis of IgG-treated podocytes via inducing autophagy. P62 and LC3B-II/LC3B-I ratio are key factors in activation of cell autophagy [40, 41]. Downregulation of p62 and increase of LC3B-II/LC3B-I could lead to the occurrence of autophagy [42, 43]. In addition, Chu et al. stated that Tim-1 could trigger autophagy in dengue virus infection [22]. Our research was in line with these previous reports, suggesting that Tim-1 could act as a promoter in LN. Meanwhile, Brooks et al. found that Tim-1-mediated T-cell proliferation was closely associated with cell autophagy [23]. Since LN is confirmed to be a type of auto-immune disease, the correlation between T-cell-mediated immune responses and autophagy in LN needs to be investigated in the future.
There are some limitations in this research. For example, some checkpoints for T cell-mediated cytokines need to be detected in LN. In addition, the correlation between Tim-1 and autophagy remains to be further explored. Hence, more investigations are needed in the future.


