Introduction
Hemostats are commonly used to control suture-hole and minor parenchymatous bleeding in all fields of surgery. As such, a wide range of materials has been introduced [1, 2]. Among these, cellulose-based hemostats (CBH) and gelatin-based hemostats (GBH) are well established and have revealed similar practicality and functionality [3–5].
Originating from polymerized glucopyranose, oxidized cellulose is fabricated in a multi-step process, generating polyuronic acid as the main structural compound through the oxidation of cellulose fibers [6]. This process makes the materials biodegradable by the human body through β-elimination and enzymatic degradation [7]. In contrast to non-regenerated cellulose, the regenerated form consists of continuous fibers with a more defined ultrastructure [8].
Gelatin is a well-established hemostatic agent and was first introduced in the 1940s. Gelatin is generated by acid partial hydrolysis from porcine-derived collagen, forming a hydrocolloid [9]. Since the implementation of the first GBH, minimal product evolution has occurred [10]. Gelatin foams can expand up to 200% and are capable of absorbing as much as 40 times their weight, which can limit their practicality in specific clinical settings [11].
Given that these hemostats are often left at the application side following hemostasis or may only be applied for short time, it is important to consider the impact of different exposure times on the local wound healing process.
Wound healing is a complex sequence of inflammation, proliferation and matrix remodeling involving various cell types [12–14]. Of these, activated fibroblasts play a pivotal role, as they synthesize an early-stage collagen-rich matrix and then differentiate to myofibroblasts to create a wound-closing tensile force [14, 15]. Therefore, fibroblast proliferation and migration are imperative to ensure physiological wound healing after surgery [16].
There is controversy as to whether hemostats interfere with post-surgical wound healing, with some evidence showing that degradation end-products might interfere with cellular sub-processes [17, 18]. As the application time of hemostats during surgery might vary from several minutes, up to weeks in those cases in which the material is left in situ, this study explores how the application time of oxidized regenerated cellulose-based (ORC), oxidized non-regenerated cellulose-based (ONRC) and GBH (GELA) alters fibroblast migration, metabolic activity and apoptosis. Further, this study analyzes changes in pH values over a 24 h time course and explores alterations of key mediating cytokines which are essential for physiological wound healing.
Material and methods
Hemostats
RESORBA CELL (RESORBA Medical GmbH, Nuremberg, Germany) (ONRC), TABOTAMP (Ethicon, Norderstedt, Germany) (ORC) and GELITA TUFT-IT (GELITA Medical, Eberbach Germany) (GELA) were used in this study. Hemostats were applied to the supernatant of fibroblast cell cultures for 5–10 minutes (min) (short-term), 30 and 60 min (intermediate-term) and continuously for 24 hours (h) (long-term).
Cell culture
Human stromal fibroblasts (PromoCell GmbH, Heidelberg, Germany) were cultivated in Dulbecco’s Modified Eagle Media (DMEM) (Biochrom GmbH, Berlin, Germany) supplemented with 20% fetal bovine calf serum (Biochrom Berlin, Germany) and 10 U/ml Penicillin/Streptomycin (PAN Biotech GmbH, Aidenbach, Germany). Fibroblasts were cultured at 37°C and CO2 5% (HERAcell240, Heraeus, Hanau, Germany) with regular media exchange. At 90% confluence, cells were sub-cultured using 0.05% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA) (PAN Biotech GmbH, Aidenbach, Germany). Passages 3 to 9 were used for experiments. Morphological cell assessment was performed using phase-contrast microscopy (Olympus CKX41, Olympus, Shinjuku, Japan).
Hemostat degradation and pH value measurement
To visualize the degradation process, hemostat sections (1 × 1 cm) were placed in 3 ml of physiological saline solution (NaCl 0.9%) (B. Braun Melsungen AG, Melsungen, Germany) or fibroblast culture media (Invitrogen/Thermo Fisher Scientific, Waltham MA USA) for 24 h. The pH value was measured at baseline, after 5, 30 and 60 min, and then hourly for the first 6 h, with additional measurements at 12 and 24 h using a pH meter (FiveEasy Profi-Kit 20 ATC, MetlerToledo, Gießen, Germany). Representative images at given time points were taken using a Discovery.V8 SteREO microscope (Carl Zeiss, Oberkochen, Germany) and/or Olympus XC10 Camera (Olympus, Shinjuku, Japan) at 30× magnification.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) for TGF-β (DY 240) and TNF-α (DY 210) were performed on fibroblast lysates according to the manufacturer’s instructions (R&D Systems, Minnesota, USA). Briefly, fibroblast cell cultures were exposed to hemostats for the stated exposure times. Next, hemostats and cell culture media were removed, and fibroblast cell cultures washed 3 times with ice-cold phosphate-buffered saline (dPBS, Invitrogen/Thermo Fisher Scientific, Waltham MA USA). Next, cell lysates were generated using RIPA buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich, Taufkirchen, Germany). Protein concentration was measured utilizing BCA-Assay (Pierce/Thermo Fisher Scientific, Waltham MA USA). Optical density was measured using a microplate reader (Victor X4, Perkin Elmer, Massachusetts, USA) with wavelengths 450 nm/570 nm.
Cell metabolic activity
Fibroblasts (1 × 105 cells/well) were seeded into a 24-well plate (Greiner Bio-One, Solingen, Germany). Hemostats were sliced into 0.5 × 0.5 cm pieces to ensure constant material input and added to the supernatant. Fibroblast cell cultures were exposed to hemostats for the stated exposure times. Thereafter, cell culture media were replaced to equal volumes. Cell metabolic activity was measured using MTT (3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) proliferation assay (Carl Roth GmbH & Co, Karlsruhe, Germany) according to the manufacturer’s instructions at baseline and after 3, 6 and 24 h of incubation. Cell culture medium was replaced with 400 µl of a 1 : 9 mixture of MTT stock solution at 5 mg/ml in dPBS (Carl Roth GmbH & Co, Karlsruhe, Germany) and culture medium. After 2 h of incubation, cells were lysed using 200 µl of 2-Propanol. Measurements were performed at a wavelength of 570 nm using a microplate reader (Victor X4, Perkin Elmer, Massachusetts, USA).
Cell migration
Suspended fibroblasts were added to both chambers of a μ-dish insert (ibidi GmbH, Planegg, Germany) and incubated for 24 h. After cell attachment, the insert was removed, and fresh culture medium was added. Hemostats were sliced into 1 × 1 cm pieces and transferred to the supernatant for 5–10, 30 and 60 min, and 24 h. After exposure, slices were removed, and culture medium was exchanged at the same volume. Representative images were taken at baseline and after 3, 6 and 24 h using a light microscope (JuLI Br, NanoEnTek, Seoul, Korea). The analysis of the images was performed using TScratch (2008, T. Gebäck and M. Schulz, ETH Zürich).
Apoptosis assay
Fibroblast apoptosis was studied using a caspase-3/7 assay following the manufacturer’s instructions (Promega, Mannheim, Germany). In short, fibroblasts were exposed to hemostats for the stated exposure times, and 100 μl of Luciferase-Mix was added. Then, the mix was incubated at room temperature for 1 h. Luciferase activity was measured using a microplate reader (Victor X4 (PerkinElmer, Massachusetts, USA).
Statistical analysis
Data are presented as mean ± SEM. Analysis was performed using GraphPad Prism 6.0 (San Diego CA, USA). Iglewicz and Hoaglin’s two-sided robust test was used to identify outliers and the modified z-score was set to 3.5. The Kolmogorov-Smirnov test was used to test for normality and 2-way ANOVA with 2-stage step-up method of Benjamini, Krieger and Yekutieli was used to test for significance. The significance level was set to p < 0.05.
Results
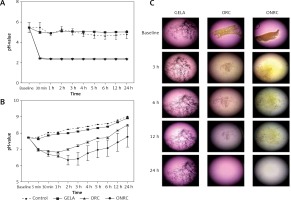
Throughout the 24 h observation period, ORC and ONRC induced lower pH values when compared to GELA and the control. ONRC more strongly acidified cell culture medium when compared to ORC (Figures 1 A, B). Specifically, both ORC and ONRC reduced pH values within 30 min in physiological saline, while GELA showed no effect on pH value when compared to control. Notably, beyond 30 min after application of ORC and ONRC the pH value stabilized (Figure 1 A). Evaluating pH values in cell culture medium, which contains substantial buffering capacity, we found that both ORC and ONRC again caused acidosis, albeit milder when compared to effects in saline. Again, GELA did not change pH values when compared to the control. After two hours of exposure, pH values started trending towards an alkaline milieu for all groups due to lower atmospheric carbon dioxide concentration (vs. incubator), suggesting no further release of acidic groups (Figure 1 B). When studying the degradation process at a macroscopic level, we observed other differences between the three hemostats. Faster degradation of ONRC compared to ORC was found after 3 h, while minimal residual material was observed after 24 h for both ORC and ONRC. Changes in pH values for both ORC and ONRC were reflected in the marked color changes of the cell culture medium. Notably GELA did not appear to degrade over time, and showed prolonged structural integrity over a 24 h time period (Figure 1 C).
Figure 1
pH values and hemostat degradation. A, B – pH values over a 24 hour time course for GELA, ORC and ONRC in saline (A) and cell culture medium (B). Recognize the immediate drop in pH value for ORC and ONRC. ONRC creates a lower pH value during the investigation period in cell culture medium vs. ORC and GELA. GELA mimicked pH values of controls in saline and cell culture medium (n = 6 samples/time point). C – Representative light microscopy images of GELA, ORC and ONRC after 3, 6, 12, and 24 hours (h) in cell culture medium. GELA was dyed with blue ink for visualization. Note the color change of the medium indicating change in pH value. Also note the structural integrity of GELA after 24 h vs. completely degraded ORC and ONRC. Original magnification: 30× (n = 6)
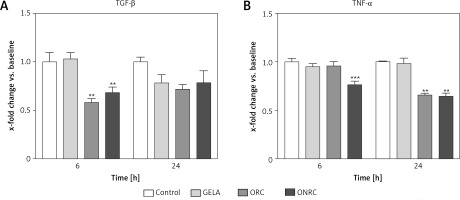
Next, we analyzed protein levels of TGF-β and TNF-α in response to hemostat exposure, given that they are key mediators for physiologic wound healing. We found that 6 h of hemostat exposure decreased TGF-β levels for ORC and ONRC significantly when compared to GELA and/or control, but this effect was not sustained to the level of statistical significance after 24 h (Figure 2 A). In contrast, TNF-α levels were decreased with ONRC exposure after 6 h and for both ORC and ONRC after 24 h of exposure (Figure 2 B). Notably, GELA did not significantly alter TGF-β or TNF-α levels at any time point (Figures 2 A, B).
Figure 2
Cytokine protein levels. Fibroblast cell cultures were exposed to hemostats for 6 and 24 hours (h). Protein levels of TGF-β (A) and TNF-α (B) were analyzed from cell lysates. ORC and ONRC reduced TGF-β levels after 6 h, while protein levels normalized after 24 h of exposure (A). ONRC reduced TNF-α levels after 6 and 24 h, while ORC reduced TNF-α levels after 24 h (B). No differential regulation vs. control was observed for GELA (A and B). **p < 0.05 ORC and/or ONRC vs. control and GELA; ***p < 0.05 ONRC vs. control, GELA and ORC. Two-way ANOVA with 2-stage step-up method of Benjamini, Krieger and Yekutieli was applied (q = 0.05, n = 4–5 samples/time point)
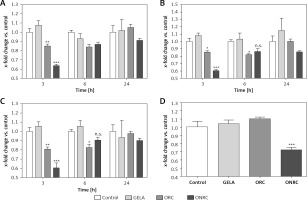
We then evaluated how different exposure times interfere with metabolic activity and migration of fibroblasts. Fibroblasts were exposed to hemostats for short- (5–10 min), intermediate- (30 and 60 min) and long-term (24 h) periods. We observed significantly reduced signal intensity during short- and intermediate-term exposure with ONRC after 3 h and with ORC after 3 h and 6 h follow-up when compared to GELA and/or control. At 24 h follow-up of the short- and intermediate-term exposures, no differences between the study groups were observed, suggesting a transient reduction of metabolic activity during ONRC and ORC exposure (Figures 3 A–C). Notably, only ONRC reduced metabolic fibroblast activity after continuous exposure for 24 h (Figure 3 D), while no changes were observed during GELA exposure at any exposure time for any follow-up time point when compared to controls (Figures 3 A–D).
Figure 3
MTT-cellular metabolic activity assay for fibroblast cell cultures with different hemostat exposure times. Data are normalized to controls. Human stromal fibroblasts were exposed to hemostats placed in their medium for 5–10 (A), 30 (B), or 60 minutes (min) (C) or continuously over 24 hours (h) (D). Cell metabolic activity was analyzed using an MTT assay after 3, 6 and 24 h. Reduced cellular metabolism was observed for both ORC and ONRC (vs. GELA and controls) at 5–10 and 60 min of hemostat exposure after 3 h (A and C). ONRC showed reduced cellular metabolism vs. control, GELA and ORC after 5–10, 30 and 60 min hemostat exposure after 3 h (A–C). This effect was maintained with continuous hemostat exposure for 24 h (D). Two-way ANOVA with 2-stage step-up method of Benjamini, Krieger and Yekutieli was applied (q = 0.05). No difference in cellular metabolism was found for GELA vs. control at any given exposure time. *p < 0.05 ORC vs. GELA; **p < 0.05 ORC vs. control and GELA; ***p < 0.05 ONRC vs. control, GELA and ORC (n = 8–12/group)
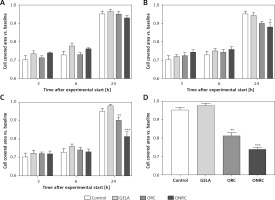
Analyzing fibroblast migration over a pre-defined gap/area between two cell fractions, we found that none of the hemostats interfered with migration after short-term exposure (Figure 4 A). In comparison, intermediate- and/or long-term exposure with ORC and/or ONRC inhibited fibroblast migration at 24 h follow-up, although shorter follow-up time points revealed no effects (Figures 4 B–D). Notably, ONRC more strongly inhibited cell migration when compared to ORC for the aforementioned exposure times (Figures 4 B, C), while GELA had no impact on fibroblast migration during any given exposure period or follow-up time point (Figures 4 A–D).
Figure 4
Fibroblast migration time course after different exposure times to hemostats. Human stromal fibroblasts were placed in both chambers of a μ-dish insert. After removal of the μ-dish insert the area between the cell fractions was measured at baseline. Hemostats were applied to the cell culture medium for 5–10 (A), 30 (B), or 60 minutes (min) (C) or continuously over 24 hours (h) (D). The area between the two cell fractions was measured after 3, 6 and 24 h from representative images. Impaired cell migration was observed for ORC/ONRC vs. GELA/control and for ORC vs. ONRC for 60 min and 24 h of exposure (C and D). *p < 0.05 vs. control; **p < 0.05 vs. control and GELA; ***p < 0.05 vs. control, GELA and ORC. Two-way ANOVA with 2-stage step-up method of Benjamini, Krieger and Yekutieli was applied (q = 0.05, n = 5–7/experimental group)
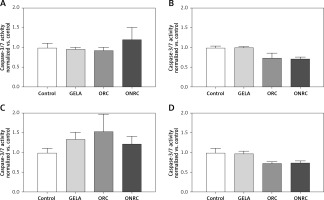
We found no relevant cell apoptosis differences in response to exposure with either ORC, ONRC or GELA vs. control (Figure 5).
Figure 5
Fibroblast caspase-3/7 activity after different exposure times to hemostats. Human stromal fibroblasts were exposed to hemostats placed in their medium for 5–10 (A), 30 (B), or 60 minutes (min) (C) or continuously over 24 hours (h) (D). Caspase-3 and -7 activity was analyzed as an indirect measurement for apoptosis. None of the applied hemostats caused significant apoptosis at the given exposure times (A–D). Two-way ANOVA with 2-stage step-up method of Benjamini, Krieger and Yekutieli was applied (q = 0.05, n = 5–7/experimental group)
Discussion
In this study we investigated the impact of different exposure times of CBH (ORC and ONRC) and GBH (GELA) on wound healing. Short-term exposure to CBH reduced cellular metabolic activity, while long-term exposure impaired cell migration. None of these unfavorable effects were observed during GBH exposure. Coincidently, CBH also acidified the local environment and altered protein levels of TGF-β and TNF-α, both key mediators of physiological wound healing [19].
Hemostats provide conformability and convenient handling, leading to these materials being integrated into daily surgical routine. As of today, there are multiple different products available, characterized by material-specific attributes. There is long-standing clinical experience with CBH and GBH, with both providing effective hemostasis and biodegradability. Despite many similarities, their mode of action differs [20, 21]. In particular, ORC and ONRC are thought to directly promote platelet activation and aggregation, forming a gel-like clot, while GBH is considered to anchor fibrinogen on the nano-rough material surface while simultaneously enhancing capillary effects [8, 22, 23]. Aside their different mode of action, hemostatic agent costs were 28–56% lower for CBH compared with other adjunctive hemostats, suggesting a major impact on overall treatment costs [24]. Indeed, current purchasing costs for GBH (GELA) appear to be higher compared with CBH (TABOTAMP > RESORBA CELL).
Our results suggest that CBH release acid groups during degradation, while GBH is pH neutral. For that reason, it might be expected that GBH lacks the anti-bacterial properties of CBH [25]. Since both CBH and GBH are bio-absorbable, they may change essential cellular wound healing processes at the site of application. Given that the application time of hemostats during surgery may or may not be limited to several minutes, we explored the impact of short-, intermediate- and long-term exposure of CBH and GBH on relevant cellular processes for physiological wound healing.
Our group and others have previously demonstrated that CBH (ORC and/or ONRC) materials cause rapid local acidosis during degradation due to the absence of buffer-acting serum components [26, 27]. Consistent with previous findings, this study found that ONRC caused the strongest decline of pH values during degradation, as frayed and less condensed fibers form a larger surface area, leading to more rapid release of acidic groups [27]. In contrast, GELA proved to be pH neutral and maintained prolonged structural integrity over 24 h. Our findings seem plausible, since reported time frames for complete degradation in vivo vary from 14 days up to 5 weeks for CBH, while the same process may take as long as 4–6 weeks for GBH and may be accelerated und standardized in in vitro conditions [28–30].
Lan et al. demonstrated that local acidosis can reduce cell metabolic activity, adhesion capacity and protein synthesis in cultured human gingival fibroblasts and described these effects as being reversible when pH values are re-adjusted to physiological conditions [31]. Similar observations have been reported for different cell types when exposed to an extracellular acidic milieu [32, 33]. The findings of the present study reveal that short-term exposure to ORC and/or ONRC significantly reduces cell metabolic activity at 3 h post-exposure, while intermediate-term and long-term exposure reduced metabolic activity at 6 h and/or 24 h, respectively. Our results do suggest that the reduction of metabolic activity for short- and intermediate-term exposure times with CBH is transient. However, GBH does not interfere with cell metabolic activity at all. Since metabolic activity is imperative for the initiation of physiological wound healing, including recruitment of various cell types and the formation of a provisional wound matrix, we conclude that GBH might be preferable when compared with CBH with regard to this endpoint [12, 34].
Along with proliferation, cell migration is crucial during the proliferative phase of physiological wound healing, as it enables the formation of granulation tissue at the side of injury [35]. Our results suggest that intermediate-term and long-term exposure to CBH impairs the migratory activity of fibroblasts at 24 h post-exposure. An association with local acidosis seems likely, since Kruse et al. demonstrated that acidic extracellular environments can impair fibroblast migration [36]. Transferring this functional finding to a cell signaling level, there is evidence that extracellular acidosis beyond the physiological range may activate mitogen-activated protein kinase (MAPK) signaling, which has shown to be involved in the regulation of cell migration [37, 38]. Given this, future experiments using targeting of MAPK signaling could help to validate our in vitro findings. For now, we again conclude that GBH might be preferred over CBH with regard to preserving fibroblast migration.
Of interest, our data suggest that some differences in functional findings may not devolve solely from acidosis, which is known to occur during CBH degradation. Our group recently found that CBH degradation end products might also cause functional alterations in migration and proliferation in fibroblasts as well as matrix contraction after exposure for 1–2 weeks [27]. Some of the phenotypic findings might have resulted from marked changes in wound healing-relevant cytokine levels. TGF-β signaling is involved in cardiovascular and pulmonary diseases [39, 40]. Denton et al. emphasized the significance of TGF-β for physiological wound healing using a mouse model of type II TGF-β receptor (TbetaRII) deletion in fibroblasts [41]. The present study suggests that, in contrast to GBH, CBH can reduce TGF-β protein levels in fibroblasts. Of interest, non-canonical intracellular TGF-β signaling might link reduced TGF-β levels upon CBH exposure to impaired fibroblast migration. In particular, it has been demonstrated that non-Smad depended signaling is able to activate the Erk MAPK pathway, which in turn interferes with cell migration [38]. Since our findings reveal delayed impaired fibroblast migration during intermediate- and long-term CBH exposure at late follow-up time points, the authors propose that evolutionarily conserved canonical TGF-β-induced Smad-signaling with interposed protein might also be critical for the findings of the present study [42–44]. Notably, short-term acidosis, such as that caused by CBH exposure, has shown to be sufficient to induce TGF-β-induced signaling [37].
Apart from TGF-β, the importance of TNF-α for physiological wound healing has been elucidated in various studies, as it promotes inflammatory leukocyte recruitment into the wounded tissues and enhances fibroblast proliferation/growth both in vitro and in vivo [45–48]. Our results indicate that CBHs can reduce intracellular TNF-α protein levels after 6 h and 24 h exposure. As a side point, these reductions in TNF-α levels might have contributed to reduced metabolic activity, which in turn serves as an indirect marker for the proliferative capacity of fibroblasts. Although reduced TNF-α levels in an acidic environment upon CBH exposure might seem contradictory at first glance, Riemann et al. also found that exposing rat kidney fibroblasts to acidic cell culture medium decreases TNF-α levels [37]. Further, since TGF-β is pivotal for the control of cell proliferation simultaneously decreased levels of TNF-α and TGF-β might have also substantially contributed to the observed impaired metabolic activity, linking the varying cytokine levels during CBH and GBH exposure to our functional findings [49, 50].
Our study has several limitations. First, we studied the effects of hemostat exposure in a monocellular in vitro model using stromal fibroblasts, which does not sufficiently mimic wound healing in vivo, as several different interacting cell types are involved. Further, the regulation of cell migration and metabolic activity through TGF-β and/or TNF-α has not been directly addressed using pathway inhibitors. Thus, a direct association between the altered cytokine levels and functional findings remains hypothetical. Lastly, it is unclear to what extent our findings are of clinical relevance, as neither experimental animal models nor human trials were part of this study.
In conclusion, this study found that ORC and ONRC (CBH) in contrast to GELA (GBH) impair essential cellular processes of physiological wound healing in vitro in fibroblasts even after short-term exposure. Decreased protein levels of TGF-β and TNF-α in response to ORC and ONRC exposure suggest that these effects may be cytokine-related. We conclude that GELA (GBH) might be more beneficial for physiological wound healing after surgery, although verification of the clinical relevance of these findings is required.