Introduction
Portal hypertension is the pivotal vascular consequence of end-stage liver disease leading to severe complications, such as variceal bleeding and ascites [1]. Transjugular intrahepatic portosystemic shunt (TIPS) is an approach that is used to alleviate portal hypertension-related symptoms including variceal bleeding, hepatorenal syndrome, refractory ascites, and hepatopulmonary syndrome [2–5]. TIPS is better able to prevent re-bleeding than endoscopic variceal ligation [5]. The TIPS procedure, however, is associated with complications including postoperative hepatic encephalopathy (HE) and TIPS dysfunction [6–10]. In prior studies, 1-year HE and TIPS dysfunction rates have been reported in the range 30–55% and 30–70%, respectively [6–10].
Covered stent use has been associated with lower TIPS dysfunction rates and decreased incidence of portal hypertensive complications in patients undergoing TIPS treatment [11, 12]. Covered stents, however, do not effectively lower postoperative HE rates [11, 12]. In some clinical trials, conducting TIPS via the left portal vein branch was suggested to be a viable approach to decreasing rates of postoperative HE [13]. In addition, other studies have found that using L-ornithine-L-aspartate may be an effective means of reducing these postoperative HE rates [14].
Postoperative HE incidence is associated with the blood volume shunted through the liver, and may thus be dependent upon stent diameter [15–21]. However, the optimal TIPS stent diameter capable of limiting HE rates remains controversial. While many studies have evaluated the relative clinical efficacy of TIPS procedures conducted using 8 mm and 10 mm stents, postoperative HE outcomes associated with these treatment approaches have varied significantly between Asian and Western studies [15–21]. Other study endpoints such as TIPS dysfunction and decreases in the portosystemic pressure gradient (PPG) have also remained controversial [15–21]. Therefore, a study which provides meta-analysis-based evidence regarding the efficacy of 8 mm vs. 10 mm stents for TIPS should be performed. In addition, the analyses based on the Asian and Western populations are also important.
Aim
This meta-analysis was thus designed in an effort to develop evidence-based treatment recommendations based upon outcomes in patients who underwent TIPS using 8 mm and 10 mm stents. We also aimed to compare the clinical efficacy of TIPS using 8 mm and 10 mm stents based on Asian and Western populations.
Material and methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement guided the conceptualization and execution of this meta-analysis. This meta-analysis was registered at the PROSPERO website (Number: CRD42020212392).
Study selection
The PubMed, Embase, and Cochrane Library databases were queried for all relevant studies published as of September 2020 using the following search strategy: (((transjugular intrahepatic portosystemic shunt[Title/Abstract]) OR (TIPS[Title/Abstract])) AND ((8 mm[Title/Abstract]) OR (small diameter[Title/Abstract]))) AND ((10 mm[Title/Abstract]) OR (large diameter[Title/Abstract])).
Studies were eligible for inclusion if they were: (a) randomized controlled trials (RCTs) or non-randomized comparative studies assessing TIPS outcomes associated with 8 mm and 10 mm stents; (b) patients with cirrhosis portal hypertension; and (c) reported ΔPPG, postoperative HE rates, TIPS dysfunction rates, re-bleeding rates, rates of liver transplantation, and/or mortality rates associated with these procedures. The languages were not limited.
Studies were excluded from this meta-analysis if they were: (a) non-comparative studies; (b) non-human studies; (c) case reports; or (d) reviews.
Data extraction
Two investigators (F.F.X. and Y. Y. W.) independently extracted key study-related data (first author, year, country, study design), patient baseline data (number and ages of patients), and TIPS-related outcome data from all studies. Any discrepancies were resolved by the corresponding author.
Quality assessment
The quality of RCTs was evaluated with the Cochrane risk of bias tool. Biases were assessed by evaluating the following items: selection, performance, detection, attrition, reporting, and other biases. Non-RCTs were evaluated with the 9-point Newcastle–Ottawa scale [22], with high-quality studies with a low bias risk being those with a score of ≥ 5 points.
Endpoints
The primary endpoint for the present meta-analysis was postoperative HE rates. The secondary endpoints for the present meta-analysis included ΔPPG, TIPS dysfunction rates, re-bleeding rates, rates of liver transplantation, and mortality rates.
Postoperative HE was defined by the detectable HE following TIPS procedure completion [16]. The diagnosis and evaluation of the degree of HE was made based on the patient’s mental state according to the West-Haven criteria [16]. Refractory HE was defined as persistence of altered mental state despite protein restriction and appropriate treatment with lactulose and/or nonabsorbable antibiotics [16]. TIPS dysfunction was defined as > 50% intra-stent stenosis, portal hypertensive complication recurrence, or a PPG > 12 mm Hg [16]. Portal hypertensive complications primarily included variceal bleeding, refractory ascites, hepatorenal syndrome, and hepatopulmonary syndrome [1–5]. Variceal bleeding was defined as the finding, at esophagogastroduodenoscopy, of ongoing or recent variceal hemorrhage or the finding of blood in the stomach and the presence of varices as the only potential cause of bleeding [18]. Refractory ascites was defined as the need for performing at least one paracentesis for ascites [18]. Hepatorenal syndrome was defined as functional renal injury developing in advanced liver disease [2]. Hepatopulmonary syndrome is defined as an arterial oxygenation defect induced by intrapulmonary vascular dilatation associated with liver disease and/or portal hypertension [3].
Statistical analysis
Statistical analyses were conducted using RevMan v5.3. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for dichotomous variables, whereas continuous variables were analyzed by estimating the pooled mean difference (MD) with corresponding 95% CIs. Heterogeneity among included studies was analyzed using χ2 tests and the I2 statistic. When analyses yielded an I2 > 50%, significant heterogeneity was considered to exist. All data were evaluated with a random-effects model. Subgroup and sensitivity analyses were conducted to probe the origin of all heterogeneity. Funnel plots were employed to gauge the risk of publication bias.
Results
Study characteristics
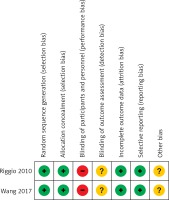
Using the search strategy detailed above, 82 potentially relevant studies were initially identified, of which seven were ultimately utilized for the present meta-analysis (Figure 1). Five of these studies were retrospective in nature [15–17, 19], while the remaining two were RCTs [18, 20]. All five of these included retrospective studies were of high quality (Newcastle-Ottawa scale scores of 6-8). The risk of bias of the RCTs is shown in Figure 2. Both of these 2 RCTs had a high risk of blinding of participants and unclear risk of blinding of outcome assessment and other bias.
These studies included in total 729 patients who had undergone the TIPS procedure, including 359 and 370 patients in the 8 mm and 10 mm stent groups, respectively (Table I). Covered stents were used in all patients, and treatment outcome data from these studies are compiled in Table II.
Table I
Characteristics of the included studies
| Study/year/country | Study design | Stent types | Groups | Sample size | Age [years] | NOS |
|---|---|---|---|---|---|---|
| Alexander/2016/USA [15] | Retrospective | Covered (VIATORR, Gore) | 8 mm | 31 | Not given | 5 |
| 10 mm | 43 | Not given | ||||
| Luo/2019/China [16] | Retrospective | Covered (FLUENCY, Bard) | 8 mm | 32 | 52 | 8 |
| 10 mm | 32 | 51 | ||||
| Miraglia/2017/Italy [17] | Retrospective | Covered (VIATORR, Gore) | 8 mm | 111 | 58.6 | 8 |
| 10 mm | 60 | 59 | ||||
| Riggio/2010/ Italy [18] | RCT | Covered (VIATORR, Gore) | 8 mm | 22 | 53.1 | – |
| 10 mm | 23 | 57.1 | ||||
| Shah/2020/USA [19] | Retrospective | Covered (VIATORR, Gore) | 8 mm | 46 | Not given | 6 |
| 10 mm | 17 | Not given | ||||
| Trebicka/2019/Germany [20] | Retrospective | Covered (VIATORR, Gore) | 8 mm | 53 | 56 | 8 |
| 10 mm | 132 | 56 | ||||
| Wang/2017/China [21] | RCT | Covered (FLUENCY, Bard) | 8 mm | 64 | 49.4 | – |
| 10 mm | 63 | 50.2 |
Table II
Characteristics of the treatment outcomes
| Study | Groups | ΔPPG [mm Hg] | HE | TIPS dysfunction | Re-bleeding | Liver transplantation | Death |
|---|---|---|---|---|---|---|---|
| Alexander [15] | 8 mm | Not given | 12/31 (38.7%) | Not given | Not given | Not given | Not given |
| 10 mm | Not given | 13/43 (30.2%) | Not given | Not given | Not given | Not given | |
| Luo [16] | 8 mm | 14.7 ±2.8 | 8/32 (25%) | 10/32 (31.3%) | 10/32 (31.3%) | Not given | 7/32 (21.9%) |
| 10 mm | 17.2 ±3.6 | 15/32 (46.9%) | 6/32 (18.8%) | 5/32 (15.6%) | Not given | 7/32 (21.9%) | |
| Miraglia [17] | 8 mm | 8.7 ±3.5 | 46/111 (41.4%) | 20/111 (18.0%) | 3/111 (2.7%) | 16/111 (14.4%) | 63/111 (56.8%) |
| 10 mm | 10.4 ±4.2 | 26/60 (43.3%) | 7/60 (11.7%) | 6/60 (10.0%) | 12/60 (20.0%) | 27/60 (45.0%) | |
| Riggio [18] | 8 mm | 12.4 ±2.2 | 11/22 (50%) | 12/22 (54.5%) | 1/22 (4.5%) | 2/22 (9.1%) | 5/22 (22.7%) |
| 10 mm | 15.2 ±3 | 11/23 (47.8%) | 3/23 (13.0%) | 0/23 (0%) | 0/23 (0%) | 3/23 (13.0%) | |
| Shah [19] | 8 mm | Not given | Not given | 10/46 (21.7%) | Not given | Not given | Not given |
| 10 mm | Not given | Not given | 5/17 (29.4%) | Not given | Not given | Not given | |
| Trebicka [20] | 8 mm | Not given | Not given | 21/53 (39.6%) | Not given | Not given | Not given |
| 10 mm | Not given | Not given | 23/132 (19.7%) | Not given | Not given | Not given | |
| Wang [21] | 8 mm | 17.2 ±1.5 | 23/64 (35.9%) | 13/64 (20.3%) | 13/64 (20.3%) | 0/64 (0%) | 13/64 (20.3%) |
| 10 mm | 17.2 ±1.5 | 32/63 (50.8%) | 10/63 (15.9%) | 10/63 (15.9%) | 1/63 (1.6%) | 17/63 (27.0%) |
Decreases in PPG
Four of the included studies provided data pertaining to ΔPPG [16–18, 20]. The decrease in PPG values in the 10 mm group was found to be significantly greater than in the 8 mm group (MD: –1.64, 95% CI: –3.17 to –0.11, p = 0.04, Figure 3 A). Significant heterogeneity was detected among these included studies (I2 = 86%).
Figure 3
Forest plots showing the comparisons in ΔPPG values (A), post-operative HE (B), TIPS dysfunction (C), re-bleeding (D), liver transplantation (E) and death between 2 groups (F)

Sensitivity analyses revealed that heterogeneity was no longer evident (I2 = 0%) when the study conducted by Wang et al. [20] was omitted from pooled analyses. Even with this study excluded, ΔPPG values remained significantly greater in the 10 mm group (p < 0.0001).
Postoperative HE
Five of the included studies provided data pertaining to rates of postoperative HE [15–18, 20], which did not differ significantly between the 8 mm and 10 mm stent groups (38.5% vs. 43.9%, OR = 0.78, 95% CI: 0.51–1.19, p = 0.25, Figure 3 B). No significant heterogeneity was detected among the included studies (I2 = 20%).
TIPS dysfunction
Data pertaining to TIPS dysfunction rates were successfully extracted from 6 studies [16–21], and the TIPS dysfunction rate was significant lower in the 10 mm group (26.2% vs. 17.4%, OR = 1.92, 95% CI: 1.14–3.23, p = 0.01, Figure 3 C). No significant heterogeneity was detected among included studies (I2 = 36%).
Re-bleeding
Four of the included studies reported outcome data pertaining to re-bleeding rates [16–18, 21], which did not differ significantly when comparing the 8 mm and 10 mm stent groups (11.8% vs. 11.8%, OR = 1.13, 95% CI: 0.40–3.17, p = 0.82, Figure 3 D). Significant heterogeneity was detected among the included studies (I2 = 53%).
Sensitivity analyses revealed that heterogeneity was no longer evident (I2 = 0%) when the study conducted by Miraglia et al. [17] was omitted from these analyses. Re-bleeding rates did not differ significantly between groups even when this study was omitted (p = 0.13).
Liver transplantation
Three of the included studies reported outcome data pertaining to rates of liver transplantation [17, 18, 21], which did not differ significantly when comparing the 8 mm and 10 mm stent groups (9.1% vs. 8.9%, OR = 0.74, 95% CI: 0.34–1.63, p = 0.45, Figure 3 E). No significant heterogeneity was detected among the included studies (I2 = 0%).
Death
Four of the included studies reported outcome data pertaining to mortality rates [16–18, 21], which did not differ significantly between the 8 mm and 10 mm stent groups (38.4% vs. 30.3%, OR = 1.20, 95% CI: 0.77–1.86, p = 0.43, Figure 3 F). No significant heterogeneity was detected among the included studies (I2 = 0%).
Publication bias
Funnel plot analyses did not suggest the existence of any potential publication bias pertaining to the selected study endpoints in the present meta-analysis.
Subgroup analyses
Subgroup analyses were performed by specifically evaluating outcomes reported in Asian (Table III) and Western (Table IV) studies. Rates of postoperative HE were found to be significantly lower in the 8 mm group relative to the 10 mm group when analyzing Asian studies (OR = 0.49, 95% CI: 0.27–0.87, p = 0.02), whereas all other endpoint data were comparable between these groups. In Western studies, ΔPPG values were significantly greater in the 10 mm group (MD: –2.16, 95% CI: –3.22 to –1.09, p < 0.0001), whereas all other endpoint data were comparable between these groups.
Table III
Meta-analytic pooled results based on the Asian studies
Table IV
Meta-analytic pooled results based on the Western studies
Discussion
In this meta-analysis, the relative safety and efficacy of TIPS procedures conducted using 8 mm and 10 mm stents were compared based upon short-term outcomes, long-term outcomes, and complication rates in these two treatment groups. Compared to a previous meta-analysis of 8 mm vs. 10 mm stents for TIPS [4], our meta-analysis has some new findings: first, a lower postoperative HE rate in Asians using 8 mm stents; second, lower post-operative PPG in Westerners using 10 mm stents.
Most of the included studies used the VIATORR stent-graft [15, 17–20]. The VIATORR stent-graft is specifically designed for TIPS allowing stent adjustment to 8, 9 or 10 mm [20, 23]. The VIATORR stent-graft has a good radial force and hoop strength. It is flexible enough to take the sharp curves sometimes encountered during creation of these shunts, making it an ideal system to be inserted into the cirrhotic liver [23]. Trebicka et al. [20] suggested dilating the VIATORR stent-graft to 8 mm as a routine, independently of the degree of ΔPPG, and revise further if necessary.
Significantly higher ΔPPG values were evident in the 10 mm group (p = 0.04). While significant heterogeneity was detected with respect to this study outcome, even when heterogeneity was eliminated through sensitivity analyses the ΔPPG values remained higher in the 10 mm group (p < 0.0001). This suggested that larger stents may be able to more effectively alleviate complications associated with portal hypertension. However, subgroup analyses suggested that greater ΔPPG values in the 10 mm group were only evident in Western studies (p < 0.0001), with no comparable difference being observed in Asian studies. A possible explanation for the pooled ΔPPG values in the Asian subgroup is that a 2 mm difference in stent diameter did not significantly alter PPG changes [24]. However, significant heterogeneity (I2 = 88%) was found in the Asian subgroup. One included study from China by Luo et al. [16] also showed a lower postoperative PPG in the 10 mm group (7.4 mm Hg vs. 9.2 mm Hg). Another included study from China by Wang et al. [21] showed a similar postoperative PPG between 2 groups (8.16 mm Hg vs. 7.35 mm Hg, p = 0.13). Therefore, further studies from Asian are still required.
Postoperative HE rates and more severe liver deterioration have previously been linked to the use of larger stents [16]. While the present meta-analysis revealed that postoperative HE rates were comparable between the 8 mm and 10 mm groups in the overall analysis (p = 0.18), subgroup analyses suggested that these rates were significantly lower in the 8 mm group when specifically analyzing Asian studies (p = 0.02). This finding might be attributed to the different body constitutions between Western and Asian patients. Moreover, stent diameter can have a significant impact on portal hepatic perfusion, and some researchers suggest that 10 mm covered stents ought to initially be dilated to 8 mm during the TIPS procedure [25, 26]. Some have further suggested that 10 mm stents only be dilated to 6–8 mm, allowing for further dilation in cases where clinical responses are found to be insufficient [27]. These recommendations are based on a model wherein under-dilated stents can maintain a lower initial caliber, thereby reducing the risk of HE.
Unlike the Asian studies in this meta-analysis, subgroup analyses of Western studies did not reveal any difference in postoperative HE rates between the two treatment groups. An RCT conducted by Riggio et al. [18] did not detect any significant reduction in HE rates or severity when using smaller stents to conduct TIPS, although this RCT ended before a large enough sample size was enrolled to detect differences in postoperative HE rates as many patients treated with smaller stents continued to suffer from significant portal hypertension complications even after undergoing TIPS [18]. In an additional retrospective study conducted by Miraglia et al. [17], postoperative HE rates were found to be similar between the 8 mm and 10 mm groups. However, the sample size was unbalanced in this study (8 mm: 111; 10 mm: 60), and future clinical trials will thus be necessary to ascertain the association between stent diameter and postoperative HE incidence.
TIPS dysfunction is a major complication that limits the long-term efficacy of TIPS intervention. We found that the use of 10 mm stents was associated with a significant increase in TIPS patency rate. This result is consistent with the finding that further reducing postoperative PPG markedly reduces the risk of TIPS dysfunction [16, 28]. However, the TIPS dysfunction rates were comparable between 2 groups based on the Asian patients (p = 0.22), and the TIPS dysfunction rate was lower in the 10 mm group, although without significance, based on the Western patients (p = 0.06). Possible explanations for these results are that: (a) most of the included studies were retrospective and selection bias existed; (b) the subgroup analysis reduced the sample size. Furthermore, we only pooled the TIPS patency rates; the HR for the TIPS patency duration was lacking in most of the included studies [15–20]. Therefore, the relationship between stent diameter and TIPS patency should be investigated in more detail.
Re-bleeding always occurred after TIPS dysfunction. However, the pooled re-bleeding rates were comparable between these groups. This result might be attributed to the fact that not all patients with TIPS dysfunction would have experienced re-bleeding.
In the previous meta-analysis performed by Liu et al. [4], the pooled liver transplantation rates were not calculated. When patients experience TIPS dysfunction or failure, liver transplantation may be employed as an alternative intervention. We observed no significant differences in liver transplantation rates between the 8 mm and 10 mm stent groups (9.1% vs. 8.9%, p = 0.27), suggesting that liver transplantation following TIPS was relatively uncommon regardless of stent size.
Mortality following TIPS is most often linked to HE, re-bleeding, or liver failure [15–20]. In this analysis, we found that post-TIPS mortality rates were comparable in the 8 mm and 10 mm stent groups (38.4% vs. 30.3%, p = 0.43). Similarly, the previous meta-analysis also demonstrated comparable pooled survival between 8 mm and 10 mm groups (p = 0.05) [4]. These findings were likely attributed to the fact that patients suffering from postoperative re-bleeding and HE generally underwent re-intervention or alternative treatment.
There are several limitations to the present meta-analysis. For one, most studies included in this analysis were retrospective in nature and are thus susceptible to selection bias. To validate these findings, additional RCTs must be conducted. In addition, we detected significant heterogeneity associated with certain study endpoints owing to variability in outcomes when comparing studies of Asian and Western patient cohorts. While subgroup analyses were conducted in an effort to evaluate and control for such heterogeneity, relatively few studies were included in this meta-analysis, limiting the value of this approach. Furthermore, some of the included studies were published in the form of conference abstracts [15, 19], limiting our ability to extract certain key pieces of data pertaining to relevant study outcomes.